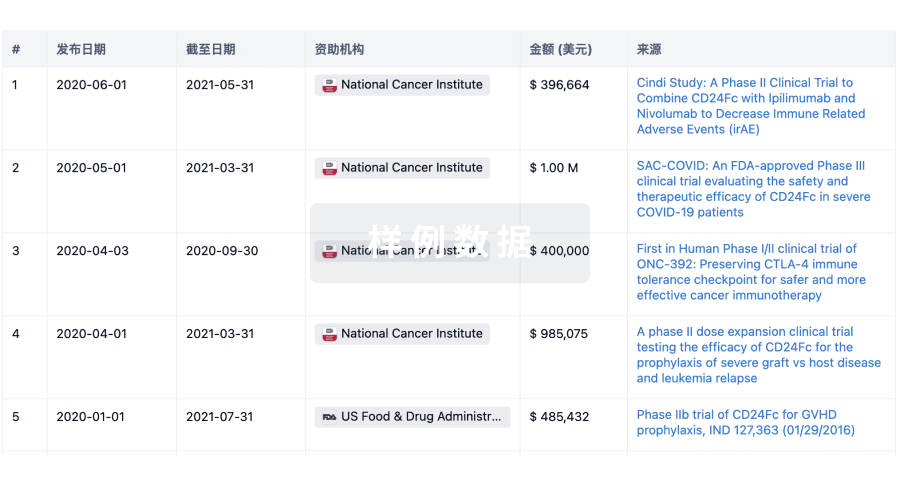
A Phase III Randomized Study Comparing the Combination of Darolutamide With Radium-223 or Placebo and the Effect on Radiological Progression-Free Survival for Patients With Metastatic Castration-Sensitive Prostrate Cancer (mCSPC)
The goal of this clinical trial is to compare the combination of Darolutamide with Radium-223 or placebo and the effects on radiological progression-free survival for patients with Metastatic Castration-Sensitive Prostrate Cancer (mCSPC)
The main questions it aims to answer are:
Radiological progression-free survival (rPFS) in mCSPC
Overall Survival (OS)
Symptomatic skeletal event-free survival (SSE-FS)
Initiation of subsequent antineoplastic therapy
Safety
Participants will have visits at baseline, treatment is once a month for up to 6 months, and long term follow up will continue until the participant dies, withdraws consent, and/or study is terminated.
Accelerated Partial Breast Irradiation (PBI) With Stereotactic Body Radiation Therapy (SBRT) or Intensity Modulated Radiation Therapy (IMRT)
This is a registry study that will be used to evaluate external beam radiation therapy methods for the accelerated treatment of breast cancer. Patients are being asked to take part in this registry because they have breast cancer and desire treatment with accelerated partial breast irradiation to be delivered by external beam methods.
Skin Cancer Oncology Radiation Evidence Registry
To collect and analyze long term safety and efficacy outcomes of patients undergoing radiotherapy for non-melanoma skin cancer. A target of 400 VMAT-treated sites is set which is estimated to be identified in approximately 350 participants. Participants referred for radiotherapy for the management of non-melanoma skin cancer.
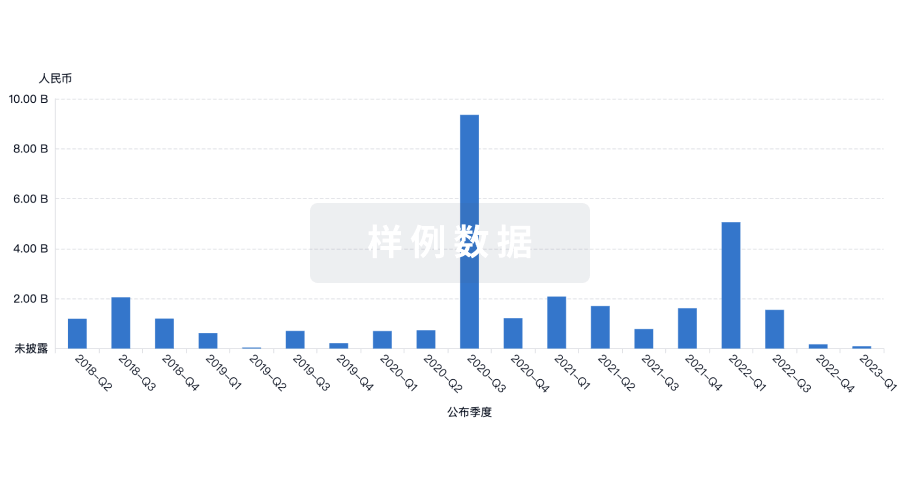
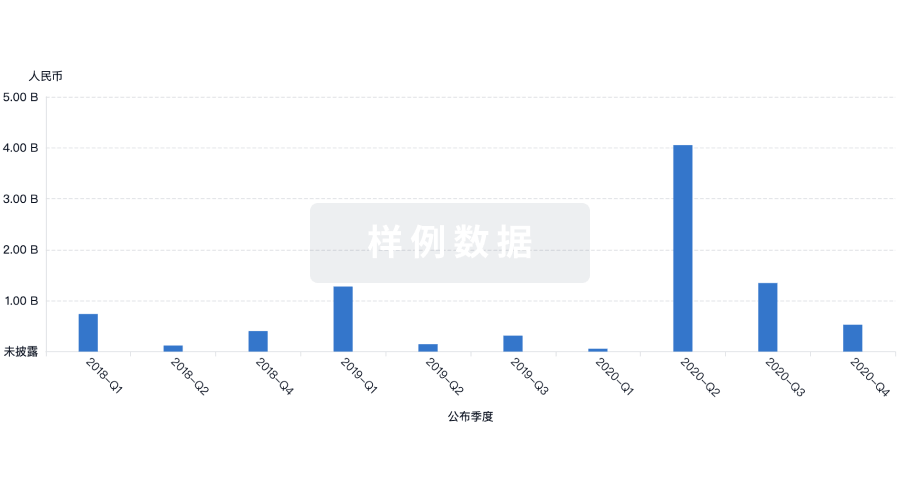
100 项与 GenesisCare USA Holdings, Inc. 相关的临床结果
0 项与 GenesisCare USA Holdings, Inc. 相关的专利(医药)
100 项与 GenesisCare USA Holdings, Inc. 相关的药物交易
100 项与 GenesisCare USA Holdings, Inc. 相关的转化医学