Incannex engages QPS for CannQuit and ReneCann
2023-04-17
并购上市批准
Preview
来源: Pharmaceutical Technology
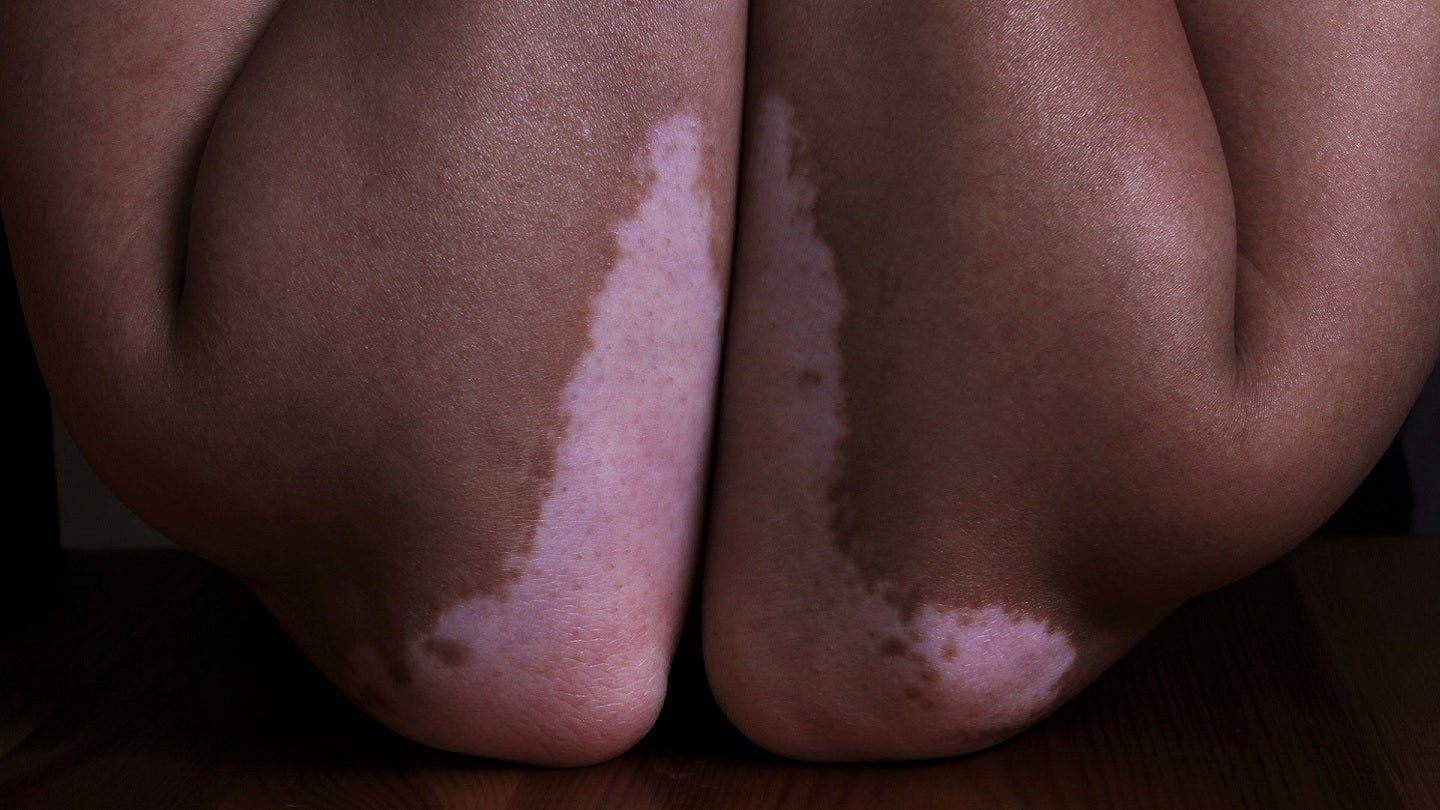
Incannex’s ReneCann is being developed to treat dermatological conditions including atopic dermatitis, psoriasis and vitiligo. Credit: Hanen BOUBAHRI on Unsplash.
Australian pharmaceutical company Incannex has appointed Quest Pharmaceutical Services (QPS) to advance CannQuit-N (Nicotine), CannQuit-O (Opioid) and Renecann Products in the USA and the European Union (EU).
QPS was established in 1995 to provide bioanalytical LC-MS/MS contract services.
The company will provide regulatory advice to Incannex, and manage clinical trials to develop CannQuit and ReneCann products to treat addiction and immune-disordered skin diseases.
QPS is preparing the pre-investigational new drug applications to be submitted to the US Food and Drug Administration (FDA) and the EU’s European Medicines Agency (EMA) for the CannQuit and ReneCann products.
The company will help in managing the clinical trials of the products once the regulators provide approvals for the proposed research and development programmes.
Incannex Healthcare CEO and managing director Joel Latham said: “Not only will QPS assist us with conducting clinical research, but it has also been engaged to advise upon the quickest route to commercialising the products in different regulatory jurisdictions.”
In 2022, the company acquired CannQuit and ReneCann products as part of the acquisition of APIRx Pharmaceuticals.
CannQuit-Nicotine combines nicotine and cannabidiol (CBD) in a controlled-release, pharmaceutical-grade medicated chewing gum formulation.
CannQuit-Opioid, named CheWell, combines CBD and an opioid antagonist/agonist in a water-soluble chewable tablet to treat opioid addiction.
ReneCann is Incannex’s topical cannabinoid formulation and is being developed to treat dermatological conditions caused by immune system disorders, including atopic dermatitis, psoriasis and vitiligo.
French company Eurofins Scientific is currently developing and manufacturing both products. It is also responsible for collecting data regarding the quality and stability of these products.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
-来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。



