预约演示
Oncotech Nordic AB AI-powered diagnostic mobile cancer screening app Ophtascan™ goes live in Central Africa
2022-10-19
LONDON, Oct. 19, 2022 /PRNewswire/ -- Medical technology innovator Oncotech Nordic AB has been awarded CE marking for the mobile health screening app, Ophtascan™, which is currently being trialled by doctors and patients in Central Africa, where there is huge demand for cancer screening and diagnosis. The new screening app is being used by Sigma Africa clinicians in the Central African Republic, Democratic Republic of Congo, Liberia and Tanzania.
Continue Reading
Preview
来源: PRNewswire
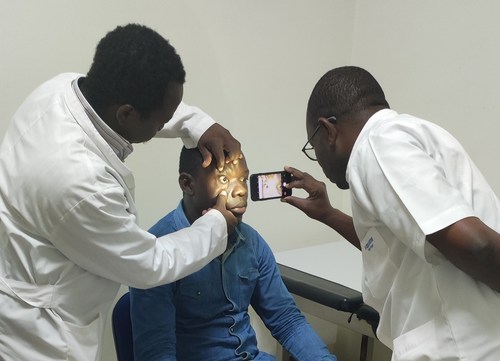
The Ophtascan™ app is used by clinicians from Sigma Africa in four countries.
The Ophtascan™ mobile phone app works by a clinician taking a high resolution photographic image of the patient's eyes, which is then analysed by Oncotech Nordic AB, making use of thousands of data sets.
The test is non-invasive, quick and requires minimal equipment, meaning that testing can take place in remote locations, far away from hospitals.
Developed since 2014 by Oncotech Nordic AB, Ophtascan™ is a mobile in-real-time detection system of pre-cancerous and all four levels of cancer conditions and targeted oncological diseases.
Clinicians can use their own smartphone devices, then photograph the iris of their patient, and upload the image to the Oncotech server. The Oncotech AI diagnostic and deep learning system then sends the results back to the clinician within just 25 seconds.
Andre Rafnsson, CEO of Oncotech said, "We are delighted to be partnering with the UK charity MedAid, which provides essential healthcare to the underprivileged in remote villages in Africa. The charity has been operating for over 20 years, providing high quality, healthcare infrastructure and services, significantly improving the lives of those living in many communities across Africa."
Simon Henry Opio-Emuna, CEO at MedAid added, "As we aim to improve health outcomes in Africa, we are excited to be working with Oncotech, who've provided us with training support on how to use the app. It's set to be a game-changer because it offers remote medical diagnosis at a very low cost per use, presenting excellent value to clinicians. It's easy to screen large numbers of patients and the test only takes a matter of seconds.
Conditions currently clinically validated and screened include lung cancer, cervical cancer, breast cancer, uterine cancer and prostate cancer."
Dr. John Paul Kaswija, a medical practitioner for Sigma Africa, a division of MedAid said, "Cancer survival rates are determined by a number of factors, and early detection is one of the most significant determinants. The diagnosis of cancer and type 2 diabetes can often be difficult because current healthcare systems require visits to hospitals, which is impractical for people in remote locations. In addition, most patients seek medical care when the disease is already advanced.
The innovative Ophtascan™ app allows clinicians with a smartphone to quickly and precisely screen patients for pre-cancerous conditions, in real-time. A bonus is that it does not unnecessarily overload the public health care diagnostic system, hence eliminating patient cancer screening waiting lists."
With its base of operations in both Sweden and the United Kingdom, Oncotech has been established by Andre Rafnsson, alongside a management team consisting of CTO Marat Kalimulov, CSO Professor Shamil Gantzev and COO Rustem Amirov.
The roots of the Ophtascan™ proof of concept technology can be traced back to post-war research and rests on ancient technology. Until recently, technology was not sophisticated enough to realise the system's full potential. In 2014, Kalimulov and Professor Gantzev relaunched the initiative by incorporating IT and AI, giving fresh impetus to the possibilities offered by an examination of the eye.
In 2022, final validation of Ophtascan™ was completed, following tests on 13,500 oncology patients over a three-year period. The scanning precision success rate was over 95 per cent, and last year reached a 97 per cent precision scanning rate, as additional patients were tested and data is validated on a daily basis.
Andre Rafnsson concluded, "MedAid is the first of what will be several telemedicine and remote health diagnostic business partners in Africa, Europe, North America and Asia with the ultimate aim being to license the Ophtascan™ technology.
In addition, new research is underway for Ophtascan™ tests that will examine the early warning signs for type 2 diabetes, a condition with rates that are seen as a 'ticking healthcare time-bomb'."
https://oncotechservices.com/
Ophtascan™ is the registered trademark of Oncotech Nordic AB.
About Oncotech Nordic AB
Oncotech works with internationally acclaimed oncologists and clinical researchers in the EMEA region.
R&D partners include:
Dana Genetics A/S, Eldon Biologicals A/S, Cencor Technology Ltd, and My Blue Label APS.
The fully funded business was set up in London, UK in 2020 with a Nordic operational subsidiary in 2021, headed by CEO Andre Rafnsson.
Photo: https://mma.prnewswire.com/media/1924734/Ophtascan_Oncotech_Nordic_AB.jpg
Logo: https://mma.prnewswire.com/media/1924733/Oncotech_Logo.jpg
SOURCE Oncotech Nordic AB
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
-药物
-Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。


