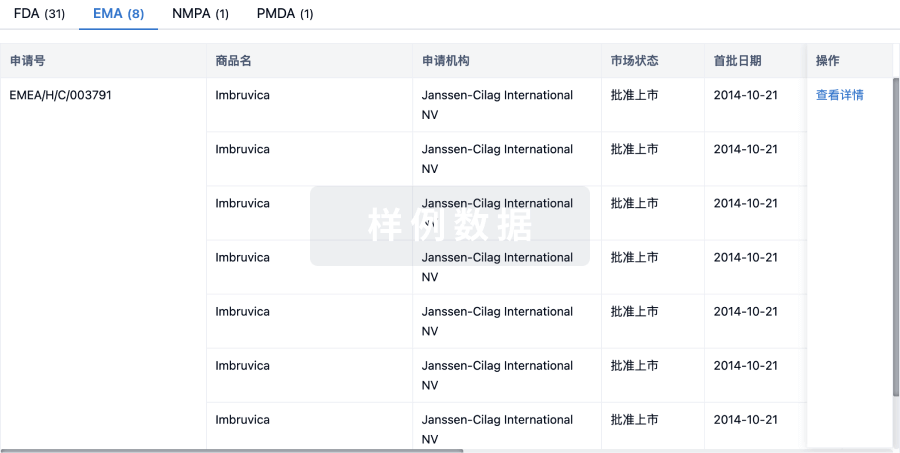
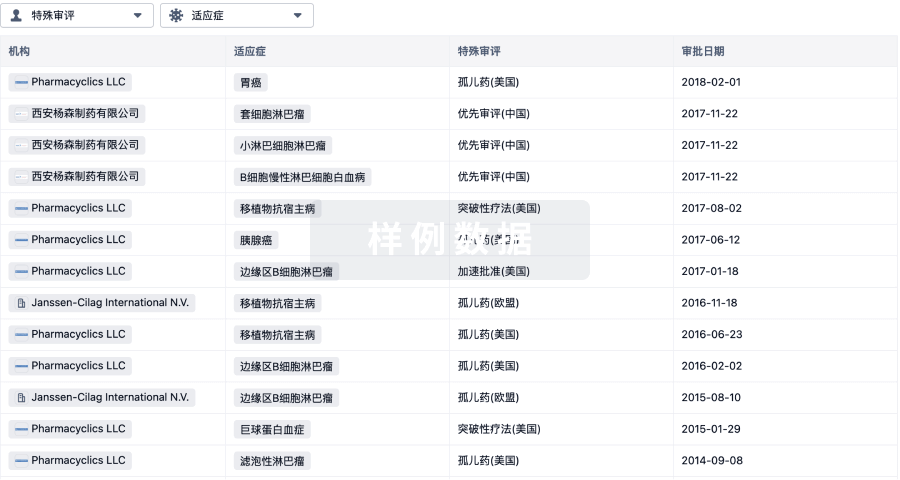
3
项与 CD30.CAR-EBVST cell therapy(Baylor College of Medicine) 相关的临床试验 / Not yet recruiting临床1期IIT Constitutive IL7R (C7R) Modified Banked Allogeneic CD30 Chimeric Antigen Receptor Epstein-Barr Virus-Specific T Lymphocytes (CD30.CAR-EBVSTs) in Patients with Relapsed or Refractory CD30-Positive Lymphomas
This study involves patients that have a cancer called diffuse large B cell lymphoma (DLBCL), Natural killer/T-cell lymphoma (NKTL), or classical Hodgkin lymphoma (cHL) (referred to collectively as lymphoma). Patients' lymphoma has come back or not gone away after treatment.
A previous research study at Baylor combined two ways of fighting disease: antibodies and T cells. Antibodies are proteins that bind to bacteria, viruses and other foreign substances to prevent disease. T-cells are special infection-fighting white blood cells that can kill tumor cells or cells infected with bacteria and viruses. Both have shown promise treating cancer, but neither has been strong enough to cure most patients. In the previous study, an antibody called anti-CD30 which is found on the surface of some T-cells and cancer cells, and had been used to treat lymphoma with limited success, was joined to the T-cells through a process called gene transfer, resulting in CD30.CAR T cells.
Another study saw encouraging responses using CD30.CAR T cells made in a lab from a patients' own blood then injected back into the same patient to treat their lymphoma. These cells are termed 'autologous' because they're given back to the original patient.
In an ongoing study, patients were treated with allogeneic CD30.CAR T cells, which are made from healthy donors instead of the patients. The use of allogenic cells avoids a lengthy manufacture time since the products are stored as a bank and available on demand. This ongoing trial has preliminarily shown promising clinical activity with no safety concerns.
With the current study, investigators plan to extend the anti-cancer effects of the CD30.CAR T cell by attaching another molecule called C7R, which has made CAR T cells have deeper and longer anticancer effects in the laboratory. The aim is to study the safety and effectiveness of allogeneic banked CD30.CAR-EBVST cells that also carry the C7R molecule, to learn the side effects of C7R modified CD30.CAR-EBVST cells in lymphoma patients, and to see whether this therapy may help them. As an extra safety step, the C7R containing T cells will also have a marker called iC9. If a patient experiences intolerable side effects from the C7R T cells, they could receive a medication called 'rimiducid' that can eliminate the C7R containing T cells by binding iC9, thereby potentially resolving the side effects. While not yet FDA approved, rimiducid has been tested in patients before without bad side effects.
A Phase 1 Study Evaluating the Safety and Activity of Allogeneic CD30 Chimeric Antigen Receptor Epstein-Barr Virus-Specific T Lymphocytes (CD30.CAR-EBVSTs) in Patients With Relapsed or Refractory CD30-Positive Lymphomas
This study involved patients that have a cancer called diffuse large B cell lymphoma (DLBCL), NK and T cell lymphomas (NK/TL) or classical Hodgkin lymphoma (cHL) (hereafter these 3 diseases will be referred to as lymphoma). Patients lymphoma has come back or not gone away after treatment. Because there is no standard treatment for the patients cancer at this time or because the currently used treatments do not work fully in all cases, the patients are being asked to volunteer in this research study.
In this study the investigators want to test a type of T cell made from a normal donor. The T cells the investigators will use are called Epstein Barr virus (EBV) specific T cells (EBVSTs) and are cells that the investigators have trained in the laboratory to recognize a EBV which is the virus that causes mono or kissing disease. Some patients with lymphoma have EBV in their cancer cells. Researchers have given T cell lines from normal donor EBVSTs to lymphoma patients who have EBV in their lymphoma cells and have seen responses in about half the patients. The cells have have been generated and are frozen in a bank. The cells are called "allogeneic" (meaning the donor is not related to the patient). CD30.CAR in EBV-specific T cells (called allogeneic CD30.CAR-EBVST) from the blood of healthy donors. The investigators are giving the cells to patients with lymphoma cells that express CD30. If the lymphoma cells also express EBV there may be some benefit from targeting both proteins.
The purpose of this study is to find out the highest safe dose of allogeneic CD30.CAR-EBVST cells given following chemotherapy and used to treat lymphoma. The investigators will learn the side effects of CD30.CAR-EBVST cells in patients and see whether this therapy may help lymphoma patients.
A Phase 1 Study Evaluating the Safety and Activity of Allogeneic CD30 Chimeric Antigen Receptor Epstein-Barr Virus-Specific T Lymphocytes (CD30.CAR-EBVSTs) in Patients with Relapsed or Refractory CD30-Positive Lymphomas
This study involved patients that have a cancer called diffuse large B cell lymphoma (DLBCL), NK and T cell lymphomas (NK/TL) or classical Hodgkin lymphoma (cHL) (hereafter these 3 diseases will be referred to as lymphoma). Patients lymphoma has come back or not gone away after treatment. Because there is no standard treatment for the patients cancer at this time or because the currently used treatments do not work fully in all cases, the patients are being asked to volunteer in this research study.
In this study the investigators want to test a type of T cell made from a normal donor. The T cells the investigators will use are called Epstein Barr virus (EBV) specific T cells (EBVSTs) and are cells that the investigators have trained in the laboratory to recognize a EBV which is the virus that causes mono or kissing disease. Some patients with lymphoma have EBV in their cancer cells. Researchers have given T cell lines from normal donor EBVSTs to lymphoma patients who have EBV in their lymphoma cells and have seen responses in about half the patients. The cells have have been generated and are frozen in a bank. The cells are called "allogeneic" (meaning the donor is not related to the patient). CD30.CAR in EBV-specific T cells (called allogeneic CD30.CAR-EBVST) from the blood of healthy donors. The investigators are giving the cells to patients with lymphoma cells that express CD30. If the lymphoma cells also express EBV there may be some benefit from targeting both proteins.
The purpose of this study is to find out the highest safe dose of allogeneic CD30.CAR-EBVST cells given following chemotherapy and used to treat lymphoma. The investigators will learn the side effects of CD30.CAR-EBVST cells in patients and see whether this therapy may help lymphoma patients
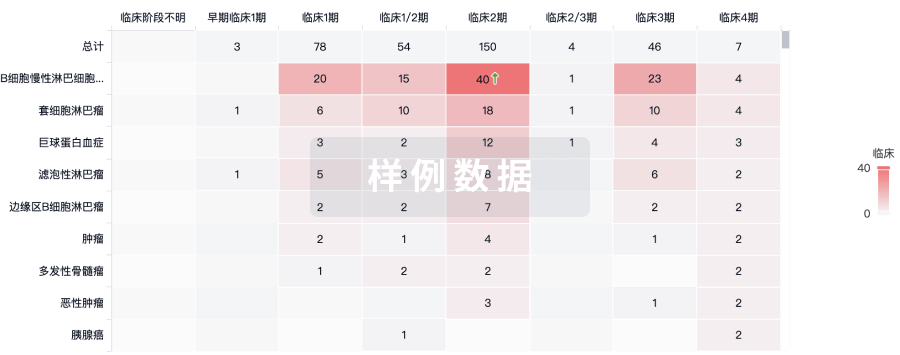
100 项与 CD30.CAR-EBVST cell therapy(Baylor College of Medicine) 相关的临床结果
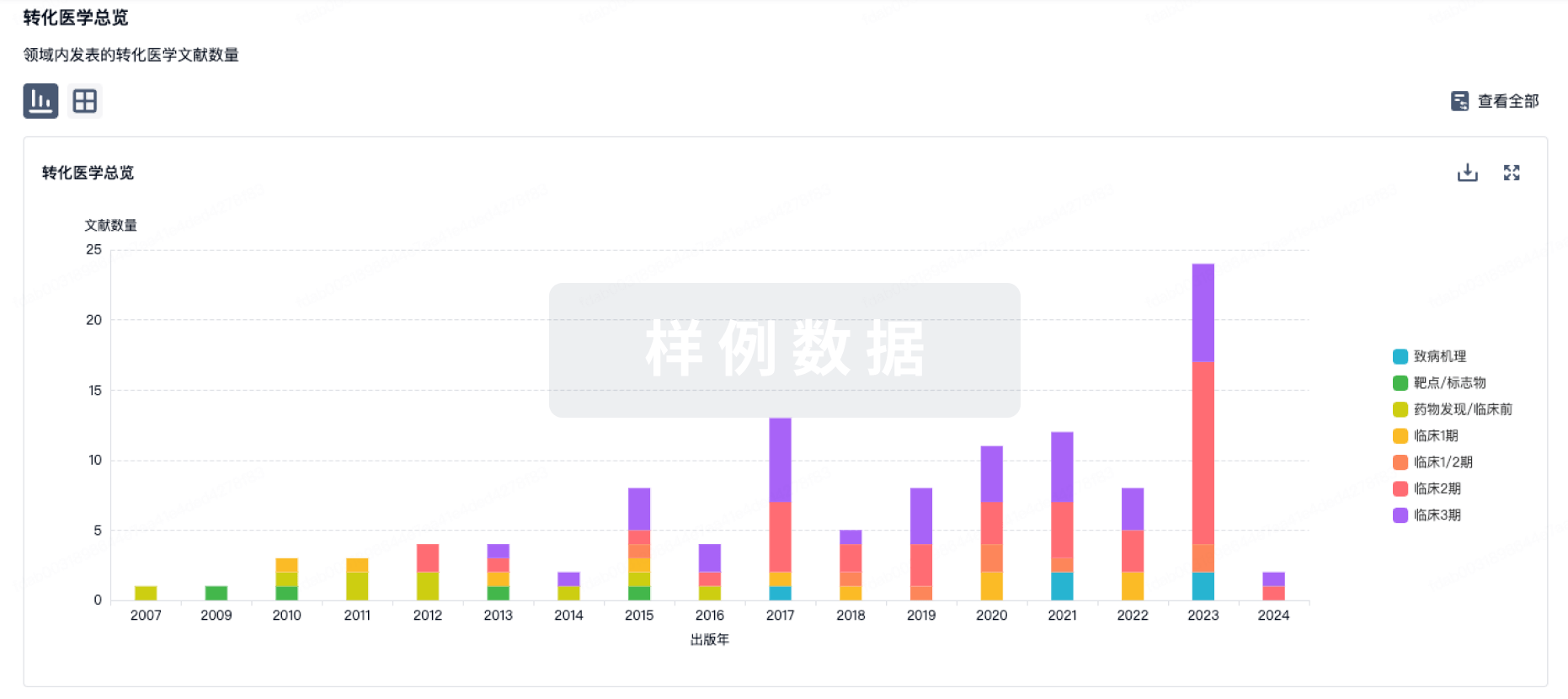
100 项与 CD30.CAR-EBVST cell therapy(Baylor College of Medicine) 相关的转化医学
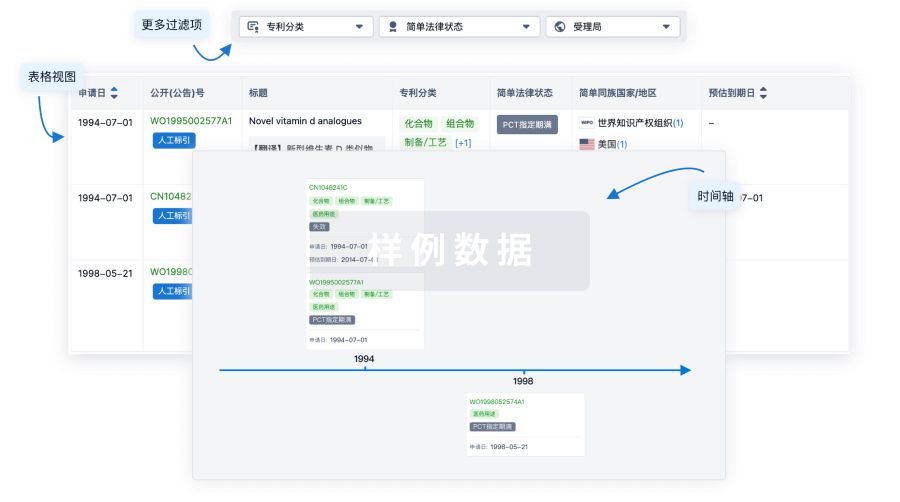
100 项与 CD30.CAR-EBVST cell therapy(Baylor College of Medicine) 相关的专利(医药)
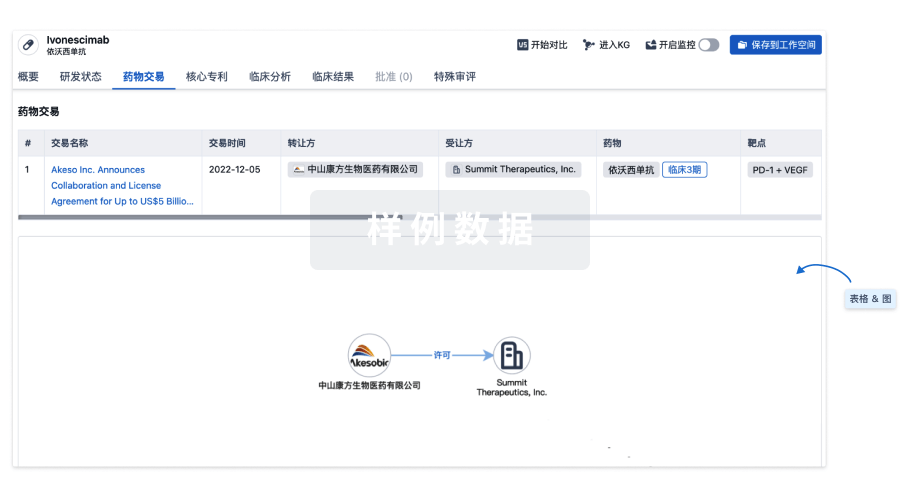
100 项与 CD30.CAR-EBVST cell therapy(Baylor College of Medicine) 相关的药物交易