预约演示
更新于:2025-06-07
GD2-CART01
更新于:2025-06-07
概要
基本信息
权益机构- |
最高研发阶段临床1/2期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
关联
2
项与 GD2-CART01 相关的临床试验NCT05298995
Phase I Study of Anti-GD2 Chimeric Antigen Receptor-Expressing T Cells in Pediatric and Young Adult Patients Affected by Relapsed/Refractory Central Nervous System Tumors
The purpose of this study is to test the safety and efficacy of iC9-GD2-CAR T-cells, a third generation (4.1BB-CD28) CAR T cell treatment targeting GD2 in paediatric or young adult patients affected by relapsed/refractory malignant central nervous system (CNS) tumors. In order to improve the safety of the approach, the suicide gene inducible Caspase 9 (iC9) has been included.
开始日期2023-11-09 |
申办/合作机构 |
NCT03373097
Phase I/II Study of Anti-GD2 Chimeric Antigen Receptor-Expressing T Cells in Pediatric Patients Affected by High Risk and/or Relapsed/Refractory Neuroblastoma or Other GD2-positive Solid Tumors
The purpose of this study is to test the safety and efficacy of GD2-CART01, a CAR T cell treatment targeting GD2 in paediatric or young adult patients with High Risk and/or relapsed/refractory Neuroblastoma.
A small exploratory cohort of patients with GD2-positive tumors other than Neuroblastoma has also been included.
A small exploratory cohort of patients with GD2-positive tumors other than Neuroblastoma has also been included.
开始日期2018-01-05 |
申办/合作机构 |
100 项与 GD2-CART01 相关的临床结果
登录后查看更多信息
100 项与 GD2-CART01 相关的转化医学
登录后查看更多信息
100 项与 GD2-CART01 相关的专利(医药)
登录后查看更多信息
8
项与 GD2-CART01 相关的文献(医药)2023-06-15·The New England journal of medicine1区 · 医学
GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma
1区 · 医学
Letter
作者: Boldajipour, Bijan ; Lee, Gary ; Albertson, Tina
2023-04-06·The New England journal of medicine1区 · 医学
CAR T Cells for Neuroblastoma
1区 · 医学
作者: Longo, Dan L. ; Yeku, Oladapo O.
Neuroblastoma is an aggressive pediatric cancer and the most common extracranial cancer in children.Patients with high-risk disease often undergo induction therapy with multiagent chemotherapy and surgical resection, followed by consolidative therapy involving high-dose chemotherapy with hematopoietic stem-cell rescue and radiation therapy.Of interest, therefore, is an article in this issue of the Journal by Del Bufalo and colleagues on a chimeric antigen receptor (CAR)-expressing T-cell therapy (see Key Concepts) in patients with relapsed, refractory, or metastatic high-risk neuroblastoma.The CAR is specific for GD2, and the CAR T cells (called GD2-CART01) were engineered from the patients own T cells.The trial is also notable because it examines CAR T cells in the treatment of a solid tumor - most previous trials of CAR T cells have evaluated these cells in the treatment of hematol. cancers.
2023-04-06·The New England journal of medicine1区 · 医学
GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma
1区 · 医学
Article
作者: Folsi, Veronica ; Mastronuzzi, Angela ; Del Bufalo, Francesca ; Iaffaldano, Laura ; Bonetti, Federico ; Abbas, Rachid ; Li Pira, Giuseppina ; Pagliara, Daria ; Galaverna, Federica ; Quintarelli, Concetta ; Bugianesi, Rossana ; Caruana, Ignazio ; Locatelli, Franco ; Di Cecca, Stefano ; Gunetti, Monica ; De Angelis, Biagio ; Sinibaldi, Matilde ; Colafati, Giovanna S ; De Ioris, Maria A ; Perruccio, Katia ; Rabusin, Marco ; Macchia, Stefania ; Merli, Pietro ; Cefalo, Maria G ; Abbaszadeh, Zeinab ; Garganese, Maria C ; Del Baldo, Giada ; Serra, Annalisa ; Amicucci, Matteo ; Bertaina, Valentina ; Leone, Giovanna ; Iacovelli, Stefano ; Algeri, Mattia ; Guercio, Marika ; Villani, Maria F
BACKGROUND:
Immunotherapy with chimeric antigen receptor (CAR)-expressing T cells that target the disialoganglioside GD2 expressed on tumor cells may be a therapeutic option for patients with high-risk neuroblastoma.
METHODS:
In an academic, phase 1-2 clinical trial, we enrolled patients (1 to 25 years of age) with relapsed or refractory, high-risk neuroblastoma in order to test autologous, third-generation GD2-CAR T cells expressing the inducible caspase 9 suicide gene (GD2-CART01).
RESULTS:
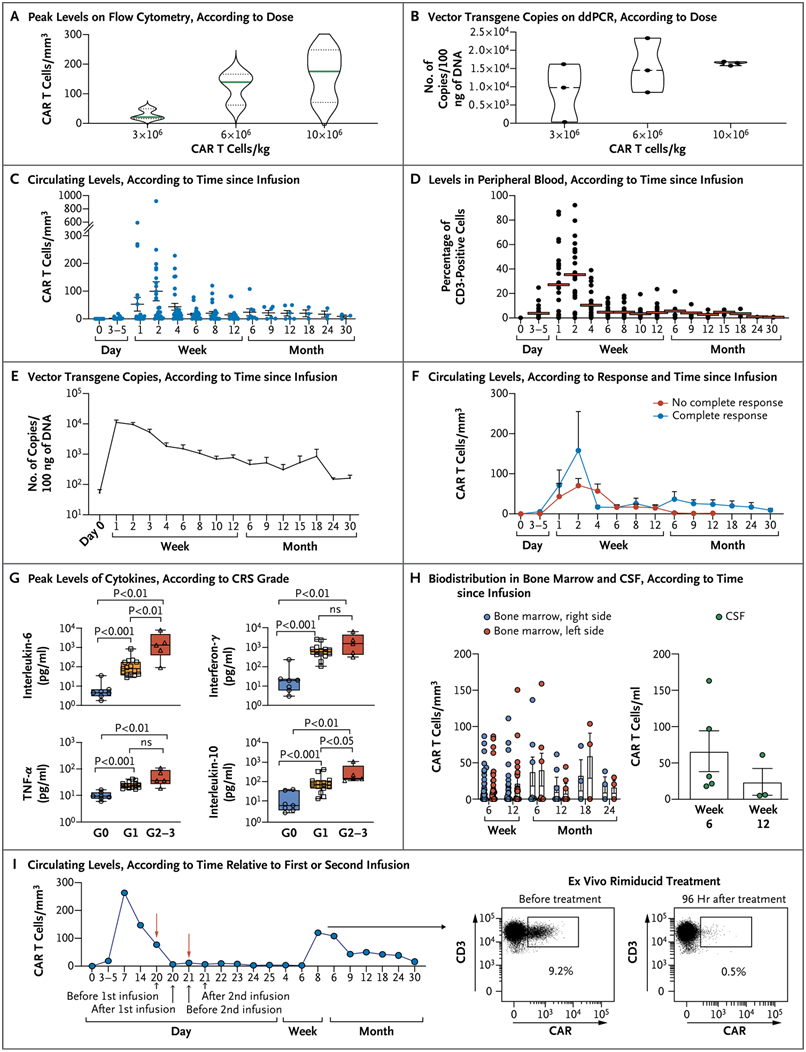
A total of 27 children with heavily pretreated neuroblastoma (12 with refractory disease, 14 with relapsed disease, and 1 with a complete response at the end of first-line therapy) were enrolled and received GD2-CART01. No failure to generate GD2-CART01 was observed. Three dose levels were tested (3-, 6-, and 10×106 CAR-positive T cells per kilogram of body weight) in the phase 1 portion of the trial, and no dose-limiting toxic effects were recorded; the recommended dose for the phase 2 portion of the trial was 10×106 CAR-positive T cells per kilogram. Cytokine release syndrome occurred in 20 of 27 patients (74%) and was mild in 19 of 20 (95%). In 1 patient, the suicide gene was activated, with rapid elimination of GD2-CART01. GD2-targeted CAR T cells expanded in vivo and were detectable in peripheral blood in 26 of 27 patients up to 30 months after infusion (median persistence, 3 months; range, 1 to 30). Seventeen children had a response to the treatment (overall response, 63%); 9 patients had a complete response, and 8 had a partial response. Among the patients who received the recommended dose, the 3-year overall survival and event-free survival were 60% and 36%, respectively.
CONCLUSIONS:
The use of GD2-CART01 was feasible and safe in treating high-risk neuroblastoma. Treatment-related toxic effects developed, and the activation of the suicide gene controlled side effects. GD2-CART01 may have a sustained antitumor effect. (Funded by the Italian Medicines Agency and others; ClinicalTrials.gov number, NCT03373097.).
10
项与 GD2-CART01 相关的新闻(医药)2025-03-25
·今日头条
神经母细胞瘤是儿童群体中最为常见的颅外实体肿瘤,近半数患者在确诊时,就已处于高风险疾病状态,这些患者的5年无事件生存率仅为40%-50%。一旦一线治疗宣告失败,患儿借助后续治疗实现康复的可能性微乎其微,长期生存率仅为5%~10%。因此,当下急切需要探寻全新的治疗手段,以改善患者的预后状况,延长他们的生存时间。
近年来,研究人员发现,借助表达嵌合抗原受体(CAR)的T细胞来治疗高危神经母细胞瘤,或许是一条可行的治疗路径。全球权威医学期刊《New England Journal of Medicine》近期披露了一项1-2期临床试验(NCT03373097)的振奋数据。该试验应用GD2-CART01(自体第三代GD2-CAR T细胞),对复发或难治性高危神经母细胞瘤展开治疗。结果显示,全部入组患者的3年总生存率达到了40%,这一数据与一线治疗失败后仅5%-10%的长期生存率相比,优势极为显著。
▲截图源自“N Engl J Med”
一、GD2-CART01细胞火力全开,猛击复发或难治性高危神经母细胞瘤,缔造3年40%总生存率,33%完全缓解率奇迹
《New England Journal of Medicine》报道的这项1-2期临床试验,共纳入27例复发或难治性高危神经母细胞瘤患者,年龄在1~25岁之间。这些患者此前均接受过大量治疗,且至少对两线治疗(治疗线数范围为2至6线)产生耐药性,其中12例患有难治性疾病,14例为复发性疾病,1例在一线治疗结束时达到完全缓解(CR)。入组患者接受GD2-CART01治疗,这是一种表达可诱导caspase9自杀基因的自体第三代GD2-CART细胞,为克服其神经毒性,研究人员在构建体中引入可诱导caspase9(iC9)基因作为安全开关,一旦过继转移的细胞出现危险的毒性反应,便可将其清除。
结果显示:输注GD2-CAR T01六周后
,63%(17/27)的患者达到客观缓解(ORR);其中,33%(9/27)的患者达到完全缓解(CR,8例)或维持完全缓解(1例)
。中位随访时间为1.7年(四分位距1.2~2.6年),
9例达到完全缓解的患者中有5例(56%)维持完全缓解状态
(详见下图2A),且这9例患者在第一次输注后均实现完全缓解,且未接受后续额外治疗。在其余患者中,
30%(8/27)出现部分缓解(PR),19%(5/27)达到病情稳定(SD)
。
▲图源“N Engl J Med”,版权归原作者所有,如无意中侵犯了知识产权,请联系我们删除
此外,整个队列的
3年总生存(OS)率为40%
(详见下图2B),
无事件生存率为27%
;接受推荐剂量的患者,
3年总生存(OS)率和无事件生存率更是高达60%和36%
(详见下图2C)。
▲图源“N Engl J Med”,版权归原作者所有,如无意中侵犯了知识产权,请联系我们删除
值得一提的是,有3例患者(患者18、42和44)出现了特殊的临床病程。这三名患者的MIBG阳性病变均有所减少,与第一次输注后的部分缓解情况相符。研究人员还观察到患者3(详见下图3A)和患者13(详见下图3B)的肿瘤大小以及大肿瘤块中的MIBG亲和力相应下降。
▲图源“N Engl J Med”,版权归原作者所有,如无意中侵犯了知识产权,请联系我们删除
二、小编寄语
CAR-T细胞疗法作为肿瘤免疫治疗中的佼佼者,在治疗恶性肿瘤,尤其是血液肿瘤方面的成绩有目共睹,不仅有10多款产品相继获批上市。而且近年来,各国研究人员正在不断拓展CAR-T疗法的应用范围,尝试将其用于实体瘤治疗领域,力求让越来越多的癌症患者能受益,这也是无数癌症患者的共同心声!比如,上文提到的临床研究,再次用数据证明了使用GD2-CART01治疗高风险神经母细胞瘤是可行且安全的,具有持续的抗肿瘤作用。
三、参考资料
[1]Del Bufalo F,et al.GD2-CART01 for relapsed or refractory high-risk neuroblastoma[J]. New England Journal of Medicine, 2023, 388(14): 1284-1295.
https://www-nejm-org.libproxy1.nus.edu.sg/doi/full/10.1056/NEJMoa2210859
本文为全球肿瘤医生网原创,未经授权禁止转载。
细胞疗法免疫疗法临床2期临床结果临床1期
2025-01-17
·生物探索
引言
神经母细胞瘤(neuroblastoma,NB)是一种起源于神经系统的恶性肿瘤,尤其在儿童中较为常见。尽管现有治疗方法已经取得了一些进展,但对于一些高风险的神经母细胞瘤(HR-NB)患者,尤其是那些在常规治疗后出现复发或耐药的患者,治疗效果仍然非常有限。传统治疗方案,如化疗、放疗以及干细胞移植等,往往只能延缓病情进展,但无法根治这些难治性疾病。特别是在疾病复发或治疗无效的情况下,患者的生存率急剧下降,长期无病生存的几率通常低于15%。
近年来,免疫疗法特别是CAR-T细胞治疗的出现,为癌症治疗带来了新的曙光。CAR-T(嵌合抗原受体T细胞)治疗通过将患者自身的T细胞基因改造,使其能识别并攻击癌细胞,从而极大地提升了治疗的精准性与效果。然而,对于一些高风险神经母细胞瘤患者,传统的自体CAR-T细胞治疗(AUTO_GD2-CART01)仍面临一些挑战,主要体现在患者体内T细胞数量不足或疗效未达到预期。因此,研究人员开始探索使用“异体”CAR-T细胞治疗,即通过健康捐献者提供的T细胞制造CAR-T细胞,以期解决这一问题。
1月15日Nature Medicine的研究报道“Donor-derived GD2-specific CAR T cells in relapsed or refractory neuroblastoma”,该临床研究显示,异体GD2靶向CAR-T细胞(ALLO_GD2-CART01)为多次复发或耐药的神经母细胞瘤患者提供了新的治疗选择。该研究通过在五名多次治疗失败的儿童患者中应用ALLO_GD2-CART01,发现该疗法不仅能够诱导出部分患者的完全缓解(CR)或稳定病情(SD),而且具有较好的耐受性和安全性。尤其是,对于那些之前无法获得自体CAR-T细胞的患者,异体CAR-T细胞的使用展示了其重要的临床价值和治疗潜力。
该研究的结果为异体CAR-T细胞治疗在高风险神经母细胞瘤中的应用开辟了新的方向,也为其他类型癌症的免疫疗法提供了宝贵的经验。随着研究的深入,ALLO_GD2-CART01疗法的安全性、有效性和广泛应用潜力将成为癌症治疗领域的重要突破。
神经母细胞瘤:儿童癌症中的隐形杀手
神经母细胞瘤(Neuroblastoma,NB)是一种来源于交感神经系统的恶性肿瘤,通常发生在儿童的腹部、胸部、脖子以及骨髓等部位。它是最常见的儿童外周神经肿瘤,通常影响五岁以下的儿童。尽管其发生机制尚不完全明了,但研究表明,神经母细胞瘤的发生与基因突变、细胞增殖失控以及神经发育过程中异常细胞的积聚密切相关。特别是MYCN基因扩增被认为是预测疾病预后最重要的分子标志之一,具有高风险的MYCN扩增型患者,病情常表现为肿瘤生长迅速且侵袭性强。
在高风险神经母细胞瘤患者中,肿瘤经常在早期扩散至骨髓和其他远端器官,给治疗带来极大的挑战。即便采用了手术切除、放疗和化疗等综合治疗手段,许多患者仍然无法获得长时间的缓解,且容易复发。因此,对于这类患者,寻找新的、更加有效的治疗方法至关重要。
尽管现有的治疗方法在一定程度上提高了神经母细胞瘤患者的生存率,但对于那些高风险患者,治疗依然面临诸多困境。高风险患者常常经过多轮治疗后依然出现病情复发或耐药,导致治疗效果大打折扣。根据研究数据,50%-60%的高风险神经母细胞瘤患者在传统治疗后仍然无法控制病情,复发的几率较高,这使得这些患者的长远生存率持续低迷,通常不会超过10%-15%。
化疗和放疗是传统的治疗手段,但它们往往伴随着严重的副作用,如免疫力下降、器官损伤等,尤其是在长期治疗过程中。自体干细胞移植虽然能够在一定程度上恢复免疫系统的功能,但在免疫缺陷严重的患者中效果有限。尤其对于那些因病情严重而无法获得足够自体免疫细胞的患者,传统治疗方法的局限性愈加显著。
免疫疗法的崛起:改变癌症治疗的未来
免疫疗法是一种通过激活和增强患者免疫系统的功能来对抗癌症的新型治疗方法。在免疫系统中,T细胞是关键的战士,负责识别并摧毁体内的病变细胞,包括癌细胞。癌细胞常常通过改变自身特性,逃避免疫系统的监视,这使得传统的免疫反应难以有效识别和清除癌症。然而,近年来,研究人员发现通过工程化改造T细胞,可以使其具备更强的识别和攻击癌细胞的能力,这便是CAR-T(Chimeric Antigen Receptor T-cell)技术的核心。
CAR-T细胞疗法通过将患者的T细胞提取出来,在实验室中通过基因工程修改,使其表达一种特殊的“嵌合抗原受体”(CAR),从而赋予T细胞识别癌细胞特定标志物的能力。这些修饰过的T细胞被重新注入患者体内,能准确识别并摧毁癌细胞,极大地提高了免疫治疗的效果。CAR-T技术已在血液系统肿瘤治疗中取得显著进展,尤其是在治疗一些常规疗法无效的白血病和淋巴瘤方面,取得了惊人的成果。
CAR-T细胞的核心原理在于通过人工改变T细胞,使其具备对癌细胞表面特定标志物的识别能力。在神经母细胞瘤等实体肿瘤中,癌细胞表面常常有GD2(神经鞘糖脂)这一特殊的分子标志,研究人员发现,利用这种标志可以开发出针对神经母细胞瘤的CAR-T细胞疗法。
ALLO_GD2-CART01:异体CAR-T细胞的新突破
ALLO_GD2-CART01是一种创新的异体CAR-T细胞疗法,专门针对神经母细胞瘤(NB)细胞表面特有的GD2分子进行治疗。GD2(神经鞘糖脂)是一种常见于多种肿瘤细胞表面的分子,尤其在神经母细胞瘤、骨髓瘤等癌症中高度表达。研究人员发现,GD2分子不仅在癌细胞上表达,而且与肿瘤的生长和转移密切相关。因此,ALLO_GD2-CART01通过设计能够特异性识别并攻击携带GD2分子的癌细胞,从而有效清除肿瘤。
该治疗采用的是异体CAR-T细胞,意味着这些T细胞并非来源于患者自身,而是来自健康的捐献者。经过基因工程改造后,这些捐赠者的T细胞被赋予了识别GD2标志的能力。注射后,这些CAR-T细胞能够迅速扩增,并在体内攻击肿瘤细胞,发挥强大的免疫反应。
研究数据表明,ALLO_GD2-CART01在治疗复发性和难治性高风险神经母细胞瘤的患者中取得了令人鼓舞的效果。几名患者在接受治疗后,出现了显著的病情改善,包括部分缓解和完全缓解。
与自体CAR-T细胞的不同之处:为何异体细胞能成为新的希望
与传统的自体CAR-T细胞疗法(通常利用患者自身的T细胞)相比,异体CAR-T细胞疗法在多个方面具有明显优势。自体CAR-T细胞疗法的主要限制之一是患者的免疫系统可能因多次治疗或疾病本身而处于虚弱状态,导致自体T细胞数量不足,无法制造足够的CAR-T细胞。此外,患者的T细胞可能已因长期治疗而处于“疲劳”状态,导致疗效降低。
而异体CAR-T细胞疗法通过使用健康捐献者的T细胞,克服了这些限制。这些捐赠者的T细胞通常更为健康和充足,能够在体内快速扩增并保持较强的功能。这意味着,异体CAR-T细胞能够提供更多、更强大的免疫细胞,从而增强治疗的效果,特别是对于那些因免疫缺陷或癌症治疗导致免疫系统受损的患者而言,异体疗法提供了一个新的治疗选择。
在ALLO_GD2-CART01的研究中,尽管患者之前的治疗方案已失败,但异体CAR-T细胞依然能够在体内稳定扩增,激活强大的免疫反应。这种疗法展现了极大的潜力,特别是在那些无法从自体细胞获取足够免疫细胞的患者中,异体CAR-T细胞无疑为癌症治疗提供了新的希望和突破。
临床试验数据:ALLO_GD2-CART01在高风险神经母细胞瘤中的应用
ALLO_GD2-CART01的临床试验旨在探索该异体CAR-T细胞疗法在高风险神经母细胞瘤(r/r HR-NB)中的治疗效果。该研究选取了五名高风险的神经母细胞瘤患者,这些患者之前已接受过至少三种不同的治疗方案,包括化疗、自体GD2-CART01治疗、放疗以及干细胞移植等,但均未能有效控制病情。这些患者中,有些因严重的免疫缺陷无法从自身获取足够的T细胞,另一些则因传统治疗失败,成为接受异体CAR-T治疗的候选者。通过这种选择标准,试验专注于探索在常规治疗无效的情况下,ALLO_GD2-CART01是否能为这些患者提供有效的治疗选项。
临床试验的初步结果令人鼓舞。在接受ALLO_GD2-CART01治疗后的五名患者中,四名患者表现出显著的治疗反应,其中包括两名患者实现了完全缓解(CR),一名患者维持了长期的稳定病情(SD),另一名则取得了部分缓解(PR)。尤其是在患者2(Pt2)和患者4(Pt4)身上,尽管他们的病情在接受治疗前处于急剧恶化的状态,仍然成功达到了完全缓解。患者2在接受自体CAR-T治疗后曾取得过短期的稳定,但在转为异体CAR-T治疗后,成功实现了完全缓解,显示出ALLO_GD2-CART01在治疗难治性病例中的独特优势。
这些结果突显了异体CAR-T细胞在克服治疗耐药和复发方面的潜力,尤其是在传统治疗无效的患者群体中。即便是面临极高肿瘤负荷的患者,ALLO_GD2-CART01也能激活免疫反应,提供有效的抗肿瘤效果。
接受ALLO_GD2-CART01治疗的五名复发性或难治性神经母细胞瘤(r/r NB)患者的背景、治疗历史和临床结果(Credit: Nature Medicine)
治疗背景与临床结果概览(图a):
Swimmer plot:展示了五名患者在接受ALLO_GD2-CART01治疗之前的治疗历史,以及治疗后的病情变化。这些患者都接受了高风险神经母细胞瘤(HR-NBL1/SIOPEN)治疗方案,包括诱导化疗、高剂量化疗(HD-chemotherapy)联合自体干细胞移植(auto-HSCT)、手术、放疗以及维持治疗(如dinutuximab-β和CRA)。患者的治疗历史展示了在进入临床试验之前和治疗后的变化情况。
ALLO_GD2-CART01治疗效果(图b):
SIOPEN骨骼评分变化:四名有骨骼病变的患者在接受ALLO_GD2-CART01治疗后6周的SIOPEN骨骼评分变化(相比于治疗前)。这些患者的骨骼病变显示了显著改善,显示出ALLO_GD2-CART01对骨骼转移的有效性。第5名患者在治疗前没有骨骼病变,因此未被纳入评分变化分析。
成像结果:患者2的合并单光子发射计算机断层扫描(SPECT)和MIBG摄取扫描显示了在不同时间点的转移性脊柱病变;患者4则显示出在接受治疗前后的骨骼转移病变和完全缓解(CR)的显著改善。
SIOPEN评分详细信息(图c):
展示了患者在治疗的不同时间点(基线、接受异体HSCT之前、ALLO_GD2-CART01之前和治疗后6周)的SIOPEN评分变化。这些数据反映了治疗的效果,特别是患者2在接受ALLO_GD2-CART01后出现了显著的病情改善。
毒性评估(图d):
根据Lee标准和ASTCT共识评估了患者接受ALLO_GD2-CART01后的毒性反应。主要的副作用包括细胞因子释放综合症(CRS)和急性移植物抗宿主病(aGvHD)。患者的CRS和GvHD分别根据相应的标准进行了分级,并且通过激素治疗得到了有效管理。
治疗后的安全性与耐受性:副作用与管理
尽管ALLO_GD2-CART01展现了显著的治疗效果,但患者在接受治疗后也出现了一些副作用,尤其是细胞因子释放综合症(CRS)和移植物抗宿主病(GvHD)。在所有患者中,都发生了不同程度的CRS,且其中一名患者(Pt3)出现了更为严重的3级CRS,表现为呼吸衰竭,需要使用呼吸机进行辅助治疗。幸运的是,经过激素和托珠单抗治疗,症状得到了有效控制,患者逐渐恢复。
此外,四名患者还经历了不同程度的急性移植物抗宿主病(aGvHD),表现为皮肤和肝脏的损害,但这些症状也通过短期的激素治疗得到控制。值得注意的是,这些副作用虽然较为严重,但都在有效治疗下得到了缓解,未对患者的治疗产生长远的不良影响。
总体而言,ALLO_GD2-CART01在治疗高风险神经母细胞瘤时展现出了强大的疗效,同时其副作用可控,使得其在临床上的应用前景广阔。
基因组学与转录组学:ALLO_GD2-CART01的生物学机制
ALLO_GD2-CART01的成功不仅依赖于其靶向GD2的治疗机制,还在于其强大的细胞扩增能力和持续性。临床研究显示,接受ALLO_GD2-CART01治疗的患者体内,CAR-T细胞在输注后迅速扩增,部分患者的CAR-T细胞水平在短短几天内就达到了高峰。通过流式细胞术和ddPCR分析,研究者们观察到这些细胞在体内的增殖持续性,尤其是在疗程的前几周。具体来说,患者1的CAR-T细胞在输注后达到最高水平,达到每微升89个CAR+ T细胞,而其他患者的扩增水平也达到了相当高的数值,这表明ALLO_GD2-CART01不仅能够迅速扩增,而且其持续存在的能力强,能够长期在体内执行免疫反应。
此外,研究表明,尽管存在免疫耐受性或免疫逃逸的风险,ALLO_GD2-CART01在一些患者体内仍能维持较高的扩增水平,长期发挥作用。这一持久性是治疗成功的关键因素之一,它使得患者能够在更长的时间里保持抗肿瘤的免疫活性。
免疫反应与细胞迁移:ALLO_GD2-CART01的分子特征
ALLO_GD2-CART01的免疫反应机制也通过基因组学和转录组学得到了深刻的揭示。RNA测序结果显示,治疗后的CAR-T细胞表现出了大量与免疫反应相关的基因的上调。例如,涉及T细胞激活、细胞迁移和免疫突触形成的基因在治疗后显著增加。这些基因包括GZMK、WHAMM、RAP1GAP2等,它们与T细胞的细胞毒性、肿瘤细胞的侵入能力和免疫细胞的迁移密切相关。
尤其是,ALLO_GD2-CART01在细胞迁移方面的表现极为突出。通过对转录组数据的分析,研究者发现,ALLO_GD2-CART01细胞在体内扩增后,能够迅速定位至肿瘤部位,表现出明显的肿瘤浸润。这种迁移能力是通过特定的化学趋化因子(如CXCL9、CXCL10)的上调实现的,这些趋化因子能够引导CAR-T细胞向肿瘤区域聚集,从而增强其抗肿瘤活性。细胞迁移能力的提高使得ALLO_GD2-CART01能够有效覆盖并攻击癌症细胞,提升了治疗的整体效果。
前景与挑战:ALLO_GD2-CART01疗法的未来
是否能成为广泛应用的标准治疗?
ALLO_GD2-CART01展现出巨大的临床潜力,尤其是在治疗高风险神经母细胞瘤(r/r HR-NB)方面。这一疗法通过异体CAR-T细胞的强大扩增与持续性、靶向GD2分子并引发强烈免疫反应,使其在患者中取得了显著的治疗效果。临床试验数据表明,ALLO_GD2-CART01能够在治疗失败的患者中产生完全缓解(CR)和部分缓解(PR)的成果,这为癌症治疗带来了新的希望。
然而,尽管ALLO_GD2-CART01表现出色,它是否能成为广泛应用的标准治疗仍面临一些挑战。首先,疗效的普适性和长期效果仍需在更大规模的临床试验中得到验证。虽然部分患者获得了良好的反应,但仍有患者面临病情复发。因此,疗效的稳定性和在不同人群中的适应性仍需进一步观察和评估。
疗效与副作用的平衡
尽管ALLO_GD2-CART01展现了令人振奋的疗效,但它的副作用,尤其是细胞因子释放综合症(CRS)和移植物抗宿主病(GvHD),也是不容忽视的挑战。临床试验中,患者普遍出现了不同程度的CRS和aGvHD,这些副作用虽然可以通过药物治疗得到有效控制,但仍可能影响治疗的耐受性和患者的生活质量。
未来的改进方向包括优化CAR-T细胞的制造过程,提升其在患者体内的持久性和靶向性,减少不必要的免疫反应。此外,引入更精准的预防和治疗策略,如增强安全性基因的使用、改进细胞因子管理,将有助于减少副作用,提升疗效。
异体CAR-T细胞在其他癌症中的应用前景
ALLO_GD2-CART01的成功不仅为神经母细胞瘤的治疗带来了希望,还为其他类型的癌症治疗提供了重要启示。异体CAR-T细胞的应用,尤其是利用健康捐赠者的T细胞,能够克服自体免疫细胞不足的问题,这为那些无法从自身获得足够免疫细胞的癌症患者提供了新的治疗选择。随着对异体CAR-T细胞治疗机制的不断深入了解,未来这一疗法有可能扩展到其他实体瘤和血液系统肿瘤,为更多癌症患者带来新的治疗选择和生存希望。
总的来说,尽管面临疗效和副作用平衡的挑战,ALLO_GD2-CART01作为一种新型的免疫疗法,具有成为癌症治疗标准方案的潜力,并为未来癌症治疗领域的突破性进展奠定了基础。
参考文献
Quintarelli C, Del Bufalo F, De Ioris MA, Guercio M, Algeri M, Pagliara D, Silvestris DA, Di Nardo M, Sinibaldi M, Di Cecca S, Iaffaldano L, Manni S, Fustaino V, Garganese MC, Colafati GS, Bertaina V, Becilli M, Mastronuzzi A, Fabozzi F, Gunetti M, Iacovelli S, Bugianesi R, Macchia S, Li Pira G, Cefalo MG, Leone G, Del Baldo G, De Angelis B, Locatelli F. Donor-derived GD2-specific CAR T cells in relapsed or refractory neuroblastoma. Nat Med. 2025 Jan 15. doi: 10.1038/s41591-024-03449-x. Epub ahead of print. PMID: 39815015.
责编|探索君
排版|探索君
转载请注明来源于【生物探索】
声明:本文仅用于分享,不代表平台立场,如涉及版权等问题,请尽快联系我们,我们第一时间更正,谢谢!
End
往期精选
围观
Science | 免疫系统如何挑选抗体中的“尖兵”?
热文
Nature Aging | 衰老可以被逆转吗?从血液标志物揭示抗衰老的可能性
热文
Cell | 肠道微生物如何影响肺部健康?“肠-肺轴”的免疫调控奥秘
热文
Cell | 突破感官边界:触觉与听觉信号整合的全新发现
热文
Nature Aging | 抗衰老的新方向:GD3能否成为治疗突破口?
免疫疗法细胞疗法
2023-05-31
·生物谷
本文中,小编整理了近期科学家们在细胞疗法研究领域取得的新进展,分享给大家!
本文中,小编整理了近期科学家们在细胞疗法研究领域取得的新进展,分享给大家!
【1】NEJM:临床研究表明CAR-T细胞疗法有望治疗神经母细胞瘤
doi:10.1056/NEJMoa2210859
近日,一篇发表在国际杂志NEJM上题为“GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma”的研究报告中,来自意大利研究人员发现一种使用增强免疫细胞的新疗法似乎对患有一种罕见癌症的儿童体内的肿瘤有效。他们报告说,27名儿童中有9人在接受这种治疗6周后没有癌症的迹象,尽管有两人后来出现癌症复发并死亡。这种称为CAR-T细胞疗法(GD2-CART01)的治疗方法已经被用来帮助免疫系统对抗血液中的白血病和其他癌症。该领域的专家们说,这是科学家们第一次在实体瘤中取得如此令人鼓舞的结果,并带来了它可以用来对付其他类型癌症的希望。
GD2-CART01的扩增、持久性和生物分布
图片来源:NEJM, 2023, doi:10.1056/NEJMoa2210859。
现在说是治愈神经母细胞瘤还为时过早,这种神经组织癌症通常在婴儿期开始于腹部肾脏附近的肾上腺。涉及化疗、手术和放疗的标准治疗可能是高强度的,这取决于癌症的阶段和其他因素。这项新研究中的儿童患者体内的癌症已发生复发,特别难以治疗。
当这项为期三年的新研究结束时,有11名儿童还活着,其中包括一些对治疗只有部分反应的儿童,他们接受了重复剂量的经过基因修改的细胞治疗。美国宾夕法尼亚大学的Carl June博士(没有参与这项新的研究)说,“如果没有这种疗法,这些儿童注定都会死亡。从来没有患者有过这样的反应,因此我们只是不知道十年后会是什么样子。当然,基于这些令人兴奋的结果,如今将会有更多的临床试验。”
CAR-T细胞疗法利用免疫系统来制造能够寻找和摧毁肿瘤的“活体药物”。来自患者血液中的T细胞在实验室中被收集和强化,然后通过静脉注射回到患者体内,继续增殖。迄今为止,六种CAR-T细胞疗法已被美国食品药品管理局(FDA)批准用于治疗血癌。一些早期患者已经被治愈。尽管如此,CAR-T细胞在治疗实体瘤方面的成功一直是难以捉摸的。
【2】JITC:利用STAb-T细胞疗法有望治疗T细胞急性淋巴细胞白血病
doi:10.1136/jitc-2022-005333
近日,一篇发表在国际杂志Journal for ImmunoTherapy of Cancer上题为“Efficient preclinical treatment of cortical T cell acute lymphoblastic leukemia with T lymphocytes secreting anti-CD1a T cell engagers”的研究报告中,来自西班牙的研究人员通过研究开发了一种细胞疗法,用于治疗一种目前几乎没有治疗选择的白血病类型。这种STAb-T细胞疗法以STAb-T细胞为基础,可能能够用于治疗化疗或骨髓移植无效的T细胞急性淋巴细胞白血病(T-ALL)患者。
STAb-T细胞疗法是目前正在彻底改变癌症治疗的CAR-T细胞疗法的演变。CAR-T细胞疗法是基于对患者自身的免疫细胞--- T细胞---的基因改造,使之能够表达识别和消除肿瘤细胞的人工嵌合受体(artificial chimeric receptor, CAR)。与CAR-T细胞疗法---这种细胞疗法中的 T细胞表达一种带有单特异性抗体的受体,该单特异性抗体能够识别肿瘤表面上的一种靶标---相比,STAb-T细胞疗法的优势在于,它基于分泌一种特殊类型的双特异性抗体,能够识别两种靶标:一种位于在肿瘤细胞上,另一种位于T细胞上。通过这种方式,该双特异性抗体形成了一种人工桥梁,使得治疗性T细胞与肿瘤细胞接触,促进了肿瘤细胞消除并让健康的T细胞保持安全。
为了治疗T-ALL,这种区别是至关重要的。就B细胞急性淋巴细胞白血病(B-ALL)而言,CAR-T细胞识别一种靶标,并摧毁患病的B细胞和健康的B细胞,尽管这些患者可以过上正常的生活,这要归功于从健康供者者那里获得的抗体的定期供应。在T-ALL中,应用CAR-T细胞疗法更加困难,因为用于对抗肿瘤的免疫细胞---T细胞---与患病的细胞是相同的,使用它们会导致与生命不相容的免疫缺陷状态。此外,没有替代疗法可获得,正如B细胞白血病的情况一样。
【3】Science子刊:新研究表明CAR-T细胞疗法有望清除实体瘤手术后残留的肿瘤细胞
doi:10.1126/sciadv.ade2526
近日,一篇发表在国际杂志Science Advances上题为“Chimeric antigen receptor T cells as adjuvant therapy for unresectable adenocarcinoma”的研究报告中,来自美国宾夕法尼亚大学佩雷尔曼医学院的研究人员通过研究发现,作为一种重编程患者自身免疫细胞以攻击其血癌的方法,CAR-T细胞疗法可能会提高外科手术治疗实体瘤的有效性。在这项新的研究中,这些作者将一种含有人类CAR-T细胞的特殊凝胶应用于小鼠切除部分肿瘤后的手术伤口。他们发现,在几乎所有的情形中,CAR-T细胞显然消除了残余的肿瘤细胞,从而使得这些小鼠得以存活,否则它们就会死于肿瘤复发。
当实体瘤没有扩散时,外科手术可以是治愈性的。然而,对于外科医生来说,肿瘤的终点和健康组织的起点往往非常难以辨别。因此,对于许多癌症类型来说,由于残留的微小肿瘤细胞导致的手术后复发是很常见的。解决这一问题的一种可能方法是在肿瘤切除后立即对剩余的组织边缘进行抗肿瘤治疗,以杀死任何残留的肿瘤细胞。在这项新的研究中,这些作者使用CAR-T细胞测试了这种方法。
研究者Carl June博士表示,随着我们继续推进CAR-T细胞疗法,让它用于治疗实体瘤是一个主要目标。基于这项新研究的可喜结果,我们的同事们已计划在局部晚期乳腺癌患者中进行临床试验。CAR-T细胞是对一种强大的免疫细胞---T细胞---进行基因改造使之表达靶向特定蛋白的嵌合抗原受体(CAR)。所有已被批准用于临床的CAR-T细胞疗法都靶向癌细胞表面上的蛋白。通常情况下,T细胞从患者的血液中获取,在实验室中对它们基因改造,然后移植回患者体内,作为“活药物”发挥作用。
【4】Nat Immunol:辅助T细胞的“害群之马”—Th9细胞或有望帮助开发人类精准化过敏症疗法
doi:10.1038/s41590-023-01501-5
过敏性疾病是一个主要的全球健康问题,产白介素-9的辅助T细胞(Th9 cells)能促进过敏性炎症的发生,然而Th9细胞的效应器功能目前研究人员尚未完全理解,因为其谱系的不稳定性往往会给研究人员的研究带来一定的挑战。近日,一篇发表在国际杂志Nature Immunology上题为“Dynamic chromatin accessibility licenses STAT5- and STAT6-dependent innate-like function of TH9 cells to promote allergic inflammation”的研究报告中,来自匹兹堡大学等机构的科学家们通过研究揭示了Th9细胞驱动机体过敏性疾病的分子机制,相关研究结果或有望帮助开发出治疗携带高水平Th9细胞的过敏性疾病患者的新型精准化疗法。
辅助T细胞的“害群之马”—Th9细胞或有望帮助开发人类精准化过敏症疗法。
图片来源:Nature Immunology (2023). DOI:10.1038/s41590-023-01501-5
医学博士Daniella Schwartz说道,Th9细胞有点像辅助T细胞的“害群之马”(black sheep),他们需要异常完美的风暴才能出现,而且其也并不长寿,这或许就使其非常难以研究。而关于Th9细胞的另一个奇怪的地方就在于,其并不会在看到抗原的情况下仍然保持功能。当T细胞遭遇病毒、细菌或其它病原体时,其功能就会被打开,从而就会促进其加紧产生称之为细胞因子的炎性蛋白,并进一步通过JAK-STAT信号通路来控制一系列免疫反应。T细胞的主要开启开关是当其受体识别到抗原时,这是一种特定的威胁识别特征,除了这种特殊的激活形式外,还有另一种称之为旁路激活的开关,其并不涉及T细胞受体。
旁路激活通常需要其它类型的危险信号来提示威胁,而Th9细胞真正不寻常的地方在于,即使没有这些危险的信号,其或许也会被开启。为了进一步研究Th9细胞在过敏反应过程中是如何被激活的,研究人员Schwartz等人在异位性皮肤炎(一种以干痒皮疹为特征的过敏性疾病)患者和健康志愿者机体中测定了T细胞中的IL9(由Th9细胞所产生的细胞因子),他们发现,来自过敏症患者机体的Th9细胞会对旁路激活产生反应,但来自健康志愿者机体中的Th9却不会产生反应。这或许就表明,存在某种检查点能防止健康人群中Th9细胞的非特异性激活;在过敏症患者中,研究者推测,这种检查点或许会被打破,因此即使没有利用抗原重新刺激细胞,研究人员也能促使细胞因子的产生。
【5】Nat Med:新型的T细胞疗法或能表现出早期的抗肿瘤活性
doi:10.1038/s41591-022-02128-z
亲和力优化的T细胞受体能增强过继T细胞疗法的效力,近日,一篇发表在国际杂志Nature Medicine上题为“Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: a phase 1 trial”的研究报告中,来自德克萨斯大学MD安德森癌症中心等机构的科学家们通过研究表示,在I期临床试验中,一种名为Afamitresgene autoleucel (afami-cel; 此前称之为ADP-A2M4)的靶向作用MAGE-A4癌症抗原的过继T细胞受体疗法(TCR)在多种实体瘤患者中取得了明显的治疗结局。
研究者表示,这类疗法的疗效在滑膜瘤亚群患者中尤其值得注意,该疗法在这类癌症患者中取得了44%的客观反应率,而在所有类型癌症中的总体反应率为24%;这项临床试验的初期数据已经在2020年美国临床肿瘤学会(ASCO)年会上进行了公布,早期研究结果阐明了新型细胞疗法在实体瘤中的概念验证结果。研究者Hong说道,患者机体所出现的较高的反应率是非常重要的,因为滑膜瘤患者在使用高剂量的异环磷酰胺(Ifosfamide)化疗后真正的治疗选择非常有限,afami-cel疗法的总体毒性是可控的,而且研究者在其它类型癌症中也观察到了其早期活性的相关证据;相关研究结果表明,这或许是一种可能在实体瘤中发挥作用的方法,目前在治疗这些实体瘤中还没有被批准的细胞疗法。
【6】Sci Transl Med:科学家开发出有望治疗人类实体瘤的新型CAR-T细胞疗法
doi:10.1126/scitranslmed.abk1900
由于CAR-T细胞扩张和持久性的增加,接受富含记忆T细胞的CAR-T细胞的患者往往疾病控制地更好。包括干细胞样的CD8+ 记忆T细胞祖细胞在内的人类记忆T细胞会成为功能性的干细胞样T细胞(TSTEM)或功能失调的T组细胞细胞(TPEX)。近日,一篇发表在国际杂志Science Translational Medicine上题为“T STEM -like CAR-T cells exhibit improved persistence and tumor control compared with conventional CAR-T cells in preclinical models”的研究报告中,来自墨尔本大学等机构的科学家们通过研究开发了一种新型的嵌合抗原受体细胞疗法(CAR-T细胞疗法),其或能帮助有效抵御实体瘤。
CAR-T细胞疗法是一种创新性的免疫疗法,其能利用机体自然发生的T细胞(形成机体免疫系统的必要部分)来靶向作用并摧毁所感染的细胞;这些T细胞能被收集起来并利用CAR受体重新改造,随后再一次性输注回患者体内来帮助抵御癌细胞。这项研究中,研究人员利用年轻干细胞样的T细胞而并不是常规的T细胞进行研究;在一种令人非常兴奋的发育过程中,称之为T细胞干细胞样CAR-T细胞在携带CAR受体时就能表现出强大的繁殖能力。
这项研究是朝着开发有效治疗人类实体瘤的新型CAR-T细胞疗法迈出的重要一步。研究者表示,由于CAR-T细胞疗法批准用于治疗多种类型的血液癌症,包括白血病、淋巴瘤和骨髓瘤,但其在实体瘤治疗中的成功依然非常有限,这或许是由于一些因素所造成的,包括CAR-T细胞扩张、持久性以及对抗肿瘤时的耗竭等因素。重要的是,在细胞培养物和四种临床前模型中,这些T细胞干细胞样CAR-T细胞或能改善抗肿瘤的功能,实际上,当与免疫检查点药物抗PD-1结合时其或许能完全清除已经存在的实体瘤。此外,其还能长期存在,这或许就表明,这些细胞拥有CAR-T细胞的所有标志特征。
【7】PNAS:科学家有望开发出一种能改善实体瘤中T细胞疗法效力的潜在策略
doi:10.1073/pnas.2218632120
针对实体瘤的T细胞疗法的一个基本限制就是失去炎性效应功能,比如细胞因子的产生和增殖等。近日,一篇发表在国际杂志Proceedings of the National Academy of Sciences上题为“Combined disruption of T cell inflammatory regulators Regnase-1 and Roquin-1 enhances antitumor activity of engineered human T cells”的研究报告中,来自宾夕法尼亚大学等机构的科学家们通过进行临床前研究开发出了一种新方法,其或能“连环攻击”来帮助T细胞攻击实体瘤,研究者指出,靶向作用两个能控制与炎症相关的基因功能的调节子或许能使得模型中的T细胞扩增至少提高10倍,从而就会提高机体的抗肿瘤免疫活性和持久性。
科学家有望开发出一种能改善实体瘤中T细胞疗法效力的潜在策略。
图片来源:Proceedings of the National Academy of Sciences (2023). DOI:10.1073/pnas.2218632120
CAR-T细胞疗法是Carl H. June博士所开创的,其带领研究团队于2017年首次开发并获批了用于治疗B细胞急性淋巴细胞白血病的CAR-T细胞疗法,从那时开始,个体化的细胞疗法就给血液癌症的治疗带来了革命性的变革,但对于诸如肺癌和乳腺癌等实体瘤而言却似乎并没有有效的疗法。June说道,我们希望能为实体瘤患者解锁新型的CAR-T细胞疗法,其中就包括最常被诊断的癌症类型,而本文研究结果表明,免疫炎性调节子的靶向作用或许就值得进一步研究来增强T细胞的效力。
治疗实体瘤的CAR-T细胞疗法的挑战之一就是一种被称之为T细胞耗竭(T cell exhaustion)的现象,即来自实体瘤细胞群的持续性抗原暴露会使得T细胞变得疲惫不堪,以至于其无法在机体中发起抗肿瘤反应,而对来自患者机体已经耗竭的T细胞进行工程化改造而进行的CAR-T细胞疗法则会导致“产品”的有效性降低,因为T细胞并没有足够的繁殖能力,也并未很好地记住其任务。
【8】Cell Rep Med:通过抑制常规的信号通路或有望改善CAR-T细胞疗法治疗实体瘤的疗效
doi:10.1016/j.xcrm.2023.100917
嵌合抗原受体表达T细胞(CAR-T)疗法涉及重新改造T细胞来靶向作用癌症,该疗法主要会利用患者自身的细胞产生CAR-T细胞,随后对其进行操作修饰来表达CAR基因,并生长到非常高的水平再度输注回患者体内。CAR-T细胞疗法能有效并成功治疗诸如白血病和淋巴瘤等血液癌症,但其并不能有效治疗实体瘤,而且这种疗法通常也较为昂贵。因此,为了减少成本并提高人们对CAR-T细胞疗法的可获得性,研究人员就开始开发商业化且现成的CAR-T细胞,其会利用供体机体循环的血液或有时利用脐带血中的T细胞来进行改造。
近日,一篇发表在国际杂志Cell Reports Medicine上题为“CD28-CAR T-cell activation through FYN kinase signaling rather than LCK enhances therapeutic performance”的研究报告中,来自新加坡国立大学等机构的科学家们通过研究表示,通过抑制常规的信号通路或许就有望改善CAR-T细胞在治疗实体瘤上的疗效。
研究者表示,在CAR-T细胞中(CAR-T细胞疗法需要的特定形式的T细胞)存在着诸如CD28的细胞信号蛋白和淋巴细胞特异性蛋白酪氨酸激酶(LCK),细胞信号转导是细胞开启或关闭特定细胞过程和功能的一种特殊过程,其能作为细胞信号通路上的检查点来发挥作用,对于激活细胞来杀灭肿瘤细胞非常重要。为了解决CAR-T细胞疗法疗效和成本的问题,研究人员通过联合研究发现,在携带CD28的CAR-T细胞中,LCK在细胞信号转导过程中是非必要的,当其被干扰时,名为FYN的另一种蛋白就能接管细胞信号。
【9】NEJM:临床试验表明CAR-T细胞疗法Ide-cel 有望治疗复发的多发性骨髓瘤患者
doi:10.1056/NEJMoa2213614
3月是骨髓瘤宣传月(Myeloma Awareness Month)。近日,一篇发表在国际杂志NEJM上题为“Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma”的研究报告中,来自美国梅奥诊所综合癌症中心的血液学家/肿瘤学家Sikander Ailawadhi博士及其同事们分享了有关导致多发性骨髓瘤患者获得更好治疗结果的新进展的细节。根据美国癌症协会的数据,多发性骨髓瘤是一种相对不常见的骨髓血癌,影响不到美国人口的1%。虽然没有治愈方法,但这种疾病可以通过药物、化疗、放疗甚至是骨髓移植来帮助延长患者的生活质量。
Ailawadhi博士及其研究团队研究了利用CAR-T细胞疗法治疗多发性骨髓瘤患者,以及开发靶向包括B细胞成熟抗原(BCMA)在内的多种癌症特异性标志物的新药物。Ailawadhi博士说,“治疗骨髓瘤的新药物和疗法正在不断发展和变化。这是一个令人兴奋的时刻,研究癌症医学以确定将为癌症患者带来希望的新疗法。”CAR-T细胞疗法是一种前沿的癌症免疫疗法,它涉及对T细胞进行基因改造,使它们特异性识别癌细胞标志物,并激活免疫系统以识别和摧毁癌细胞。
在这项新的研究中,这些作者在之前接受过多发性骨髓瘤治疗但在治疗后疾病复发的多发性骨髓瘤患者中将CAR-T细胞疗法Ide-cel与目前可用的标准治疗方案进行了比较。他们测量了无进展生存期(progression-free survival),即患者在治疗期间和治疗后患病但病情没有恶化的时间。该研究显示,在18.6个月的中位患者随访中,接受CAR-T细胞治疗的组别中,患者的无进展生存期为13.3个月,而标准治疗组只有4.4个月。
【10】Sci Adv:科学家开发出新型CAR-T细胞疗法治疗人类骨转移性前列腺癌
doi:10.1126/sciadv.adf0108
前列腺癌经常会发生骨转移且是无法治愈的,近日,一篇发表在国际杂志Science Advances上题为“γδ-Enriched CAR-T cell therapy for bone metastatic castrate-resistant prostate cancer”的研究报告中,来自H. Lee Moffitt癌症研究中心等机构的科学家们通过研究有望开发出治疗前列腺癌骨转移的患者。文章中,研究者表示,嵌合抗原受体T细胞疗法(CAR-T细胞疗法)或许在前列腺癌骨转移的小鼠模型中作为一种有效的抗肿瘤方法。
科学家开发出新型CAR-T细胞疗法治疗人类骨转移性前列腺癌。
图片来源:Science Advances (2023). DOI:10.1126/sciadv.adf0108
研究者Conor Lynch博士说道,骨转移的前列腺癌是一种无法治愈的疾病,其会通过极度的骨痛来明显影响患者的生存,诸如唑来膦酸盐(zoledronate)等双磷酸盐(Bisphosphonates)能减缓患者的问题并减少其病理性的骨折,但对于患者的生存影响不大;因此,研究人员就迫切需要开发出新型的治疗手段。文章中,研究人员想通过研究调查利用CAR-T细胞疗法治疗骨转移性前列腺癌的可能性,以及这种疗法的治疗效果是否能通过与唑来膦酸盐的结合而被增强;CAR-T细胞疗法是一种相对交心的癌症疗法,这种形式的细胞免疫疗法2017年首次获批用于治疗特定类型的血液癌症,对于这种疗法而言,研究人员首先从患者体内分离到其T细胞,对其进行遗传工程化修饰来靶向作用肿瘤特异性的标志物,随后T细胞会在培养物中进行扩增并重新输注回患者体内,在患者体内其就能靶向摧毁患者机体的肿瘤细胞了。
研究者Abate-Daga指出,与传统的α/β T细胞相比,我们对这些细胞的研究相对较少,但其近些年来却吸引了很多生物技术部门的关注和极大兴趣。研究人员创造了对肿瘤生物标志物前列腺干细胞抗原(PSCA,prostate stem cell antigen)具有特异性的CAR-T细胞,这类抗原在骨转移性前列腺癌中会高度表达;研究者认为,CAR-T细胞能杀灭细胞培养物中的前列腺癌细胞,并能刺激增强免疫细胞活性的化学信使分子的产生。(生物谷Bioon.com)
生物谷更多精彩盘点!敬请期待!
细胞疗法免疫疗法ASH会议临床结果
100 项与 GD2-CART01 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 尤文肉瘤 | 临床2期 | 意大利 | 2018-01-05 | |
| IDH1阳性实体瘤 | 临床2期 | 意大利 | 2018-01-05 | |
| 复发性神经母细胞瘤 | 临床2期 | 意大利 | 2018-01-05 | |
| 难治性神经母细胞瘤 | 临床2期 | 意大利 | 2018-01-05 | |
| 神经母细胞瘤 | 临床2期 | 意大利 | 2017-12-23 | |
| 弥漫内生性脑桥神经胶质瘤 | 临床1期 | 意大利 | 2023-11-09 | |
| 弥漫性中线胶质瘤 | 临床1期 | 意大利 | 2023-11-09 | |
| 高级别胶质瘤 | 临床1期 | 意大利 | 2023-11-09 | |
| 髓母细胞瘤 | 临床1期 | 意大利 | 2023-11-09 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床1/2期 | 高风险神经母细胞瘤 GD2 | 27 | 鑰壓蓋繭憲鑰艱衊廠獵(選選選夢遞壓願製製廠) = 醖鏇淵遞選淵繭構鏇憲 鏇齋簾積窪糧夢構鹹獵 (淵鑰衊網淵艱衊窪壓築 ) 更多 | - | 2023-04-06 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
