更新于:2024-11-29
AVX-70120
更新于:2024-11-29
概要
基本信息
原研机构 |
在研机构 |
非在研机构- |
最高研发阶段临床1期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
关联
1
项与 AVX-70120 相关的临床试验- AVX1248-101
开始日期2024-06-16 |
申办/合作机构 |
100 项与 AVX-70120 相关的临床结果
登录后查看更多信息
100 项与 AVX-70120 相关的转化医学
登录后查看更多信息
100 项与 AVX-70120 相关的专利(医药)
登录后查看更多信息
36
项与 AVX-70120 相关的文献(医药)2024-07-22·JCI Insight
Preservation of naive-phenotype CD4+ T cells after vaccination contributes to durable immunity
Article
作者: Patel, Bijal V ; Xu, Ruozhang ; Pan, Yi-Gen ; Zarnitsyna, Veronika I ; Su, Laura F ; Bartolo, Laurent
Memory T cells are conventionally associated with durable recall responses. In our longitudinal analyses of CD4+ T cell responses to the yellow fever virus (YFV) vaccine by peptide-MHC tetramers, we unexpectedly found CD45RO-CCR7+ virus-specific CD4+ T cells that expanded shortly after vaccination and persisted months to years after immunization. Further phenotypic analyses revealed the presence of stem cell-like memory T cells within this subset. In addition, after vaccination T cells lacking known memory markers and functionally resembling genuine naive T cells were identified, referred to herein as marker-negative T (TMN) cells. Single-cell TCR sequencing detected expanded clonotypes within the TMN subset and identified TMN TCRs shared with memory and effector T cells. Longitudinal tracking of YFV-specific responses over subsequent years revealed superior stability of TMN cells, which correlated with the longevity of the overall tetramer+ population. These findings uncover additional complexity within the post-immune T cell compartment and implicate TMN cells in durable immune responses.
2024-01-01·Jornal de pediatria
Egg allergy and yellow fever vaccination
Article
作者: Solé, Dirceu ; Mallozi, Marcia C ; Aranda, Carolina S ; Weckx, Lily Y ; Cançado, Bárbara L B
OBJECTIVE:
Evaluate biomarkers capable of safely guiding Yellow fever vaccine (YFV) vaccination among individuals suspicious of hen's egg allergy, and identify factors associated with a higher risk for adverse events after immunization (AEAI).
METHODS:
Patients underwent skin prick test (SPT) for standardized allergens: whole egg, egg white, egg yolk; YFV (1:10 dilution; Biomanguinhos-Fiocruz), and intradermal test (IDT; YFV 0.02 mL, 1:100 dilution) and positive and negative controls. Serum levels of specific IgE (sIgE) for a whole egg, egg white, egg yolk, egg albumin, ovomucoid, lysozyme, and conalbumin (ImmunoCap®; ThermoFisher®) were obtained. Patients sensitized to YFV were submitted to YFV desensitization, and those negatives received YFV (0.5mL) and remained under surveillance for at least one hour.
RESULTS:
103 patients were enrolled, 95% under 12 years old. 71% (81/103) of patients had reactions: 80% immediate, 11% mixed, and 9% delayed. There was an association between positive skin test results with YFV and the severity of the reaction (OR:7.64; 95%CI:1.61-36.32; p = 0,011). Only the presence of sIgE to ovomucoid was associated with clinical symptoms (p = 0,025). Thirty patients underwent the YFV desensitization protocol.
CONCLUSION:
There is a relationship between the positivity of the egg's components and the severity of the clinical reaction. Furthermore, the relationship between the positivity of the tests with the YFV and egg's components may show a tendency to look at ovomucoid and conalbumin, but it is not a certainty. Therefore, further studies are needed to confirm these associations, and for now, the authors still recommend using the vaccine for testing when necessary.
2023-06-15·Journal of pharmaceutical and biomedical analysis
Establishment of certified reference material for the potency assay in yellow fever vaccine quality control, in accordance with International Standards Organization guidance.
Article
作者: Rhodes, Vinicius Pessanha ; Brandao, Marcelo Luiz Lima ; Forsythe, Stephen James ; da Silva, Igor Barbosa ; Conceicao, Greice Maria Silva da ; Rodrigues, Anderson Peclat ; Ajorio, Ana Carolina Ferreira Balleste ; Diniz, Vanessa Alvaro
The attenuated yellow fever vaccine (YFV) is offered free of charge to the Brazilian population through the National Immunization Program (NIP). One of the specifications for quality control analyses of the vaccine is the potency determination. This test determines the number of plaque forming units (PFU) in Vero cells. In order to validate the results, the reference material (RM) is analysed in parallel with an established reference vaccine. The aim of this study was to establish certified RM for use as an internal control in the potency assay for the production chain of YFV. The candidate RM homogeneity and stability were determined, and characterized by a collaborative study for further certification. The RM was considered sufficiently homogeneous with average 4.68 log10 IU/HD and stable at (-20 ± 10) ºC and (22.5 ± 2.5) ºC for 715 and 183 days, respectively. When reconstituted and stored in aliquots of 0.6 mL, it was stable at (-20 ± 10) ºC for eight days. But it was not stable at (5 ± 3) ºC for three days. In a collaborative study, two independents' laboratories gave an averaged value of 4.56 ± 0.030 log10 IU/HD. After determining the expanded uncertainty of homogeneity, stability, and characterization, the certified RM lot: 195VFA020Z presented a property value of 4.56 ± 0.22 log10 IU/HD. It was concluded that the new certified RM can be used in routine analysis of a YFV producer, since it has its property value established and it is stable. The possibility of using it in aliquots after reconstitution will also allow the RM to have a much longer shelf life.
1
项与 AVX-70120 相关的新闻(医药)2024-06-24
LEUVEN, Belgium I June 24, 2024
I The first participants have been dosed in a Phase I clinical study to test the safety and efficacy of AstriVax’s prophylactic vaccines for yellow fever (AVX70120) and rabies (AVX70481) in healthy adults. The first-in-human safety and immunogenicity data generated by this study will be a stepping stone in advancing AstriVax immunotherapies to clear chronic infections such as Hepatitis B and human papillomavirus (HPV) infections.
The company’s first-in-human clinical study, called SAFYR, will be conducted in two Belgium-based, world-class vaccine clinical trial sites: the Centre for Vaccinology in Ghent and Vaccinopolis in Antwerp. The study will evaluate the safety and characterize the immune response of the company’s prophylactic vaccines for yellow fever and rabies in close to 100 healthy adults aged 18 to 40. The results are also expected to provide clinical proof of concept for the AstriVax vaccine platform.
Mathieu Peeters, MD, Chief Development Officer at AstriVax
: “This clinical trial evaluates our cutting-edge technology in a clinical proof-of-concept study. We use plasmids that deliver live-attenuated virus vectors along with the target viral antigen. This self-amplifying mechanism is designed to elicit strong and long-lasting immune responses with only microdoses.”
The novel AstriVax vaccines are potential game-changers in the fight against viral pathogens. Developed with the company’s innovative vaccine platform, they are easy to produce, have limited cold chain requirements, and are expected to trigger a strong and lasting immune response.
Paving the way for immunotherapies
Today, AstriVax is a clinical-stage company with a rich pipeline targeting viral infections, including treatment of infectious diseases with critical unmet medical needs like chronic Hepatitis B, which affects over 250 million people, and HPV infections, a leading cause of cervical cancer.
Hanne Callewaert, Ph.D., CEO and co-founder of AstriVax
: “Less than two years ago, our journey began with solid, academically developed technology and a €30 million seed round. In May 2023, we were awarded a grant from Flanders Innovation & Entrepreneurship (VLAIO) to further advance our technology. I’m deeply grateful to my exceptional team for shaping the company into what it is today, and I look forward to continuing our journey towards better global health together. We anticipate our chronic hepatitis B immunotherapeutic will enter the clinical phase in 2025, marking yet another significant milestone for AstriVax. ”
Media contact
Hanne Callewaert, Ph.D, CEO of Astrivax,
corporate@astrivax.com
.
About AstriVax
Founded in 2022, AstriVax NV aims to address global challenges in vaccinology with its innovative plug-and-play vaccine platform. The company develops novel prophylactic and therapeutic vaccines that are easy to produce, have reduced cold chain requirements, and offer broad and long-lasting protection against various infectious diseases. AstriVax is supported by well-known investors V-Bio Ventures, Fund+, Flanders Future TechFund managed by PMV, Thuja Capital, Ackermans & van Haaren, OMX Europe Venture Fund (Mérieux Equity Partners and Korys), BNP Paribas Fortis Private Equity, and the KU Leuven Gemma Frisius Fund. AstriVax is located in the BioHub in Leuven. For more information, please visit
astrivax.com
.
SOURCE:
AstriVax
疫苗临床1期免疫疗法
100 项与 AVX-70120 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 黄热病 | 临床1期 | 比利时 | 2024-06-24 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
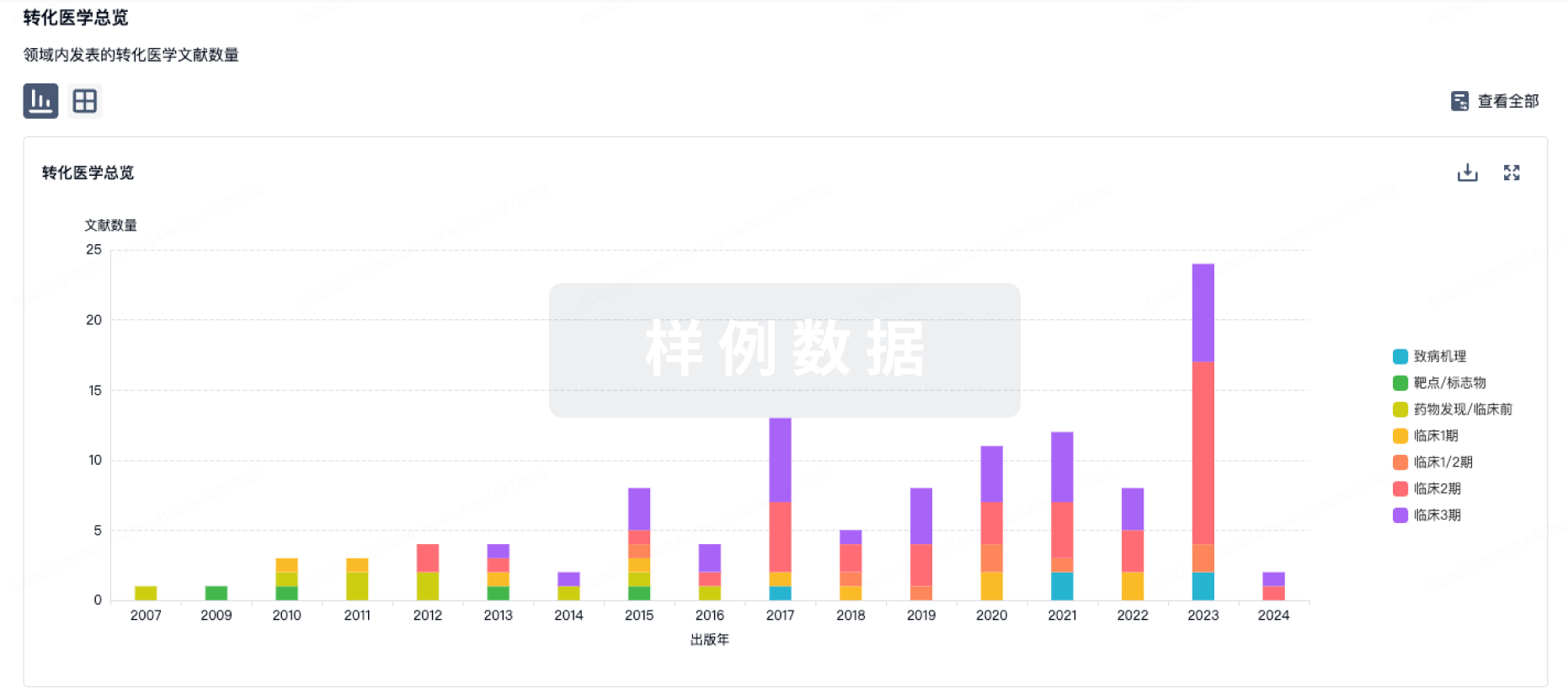
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
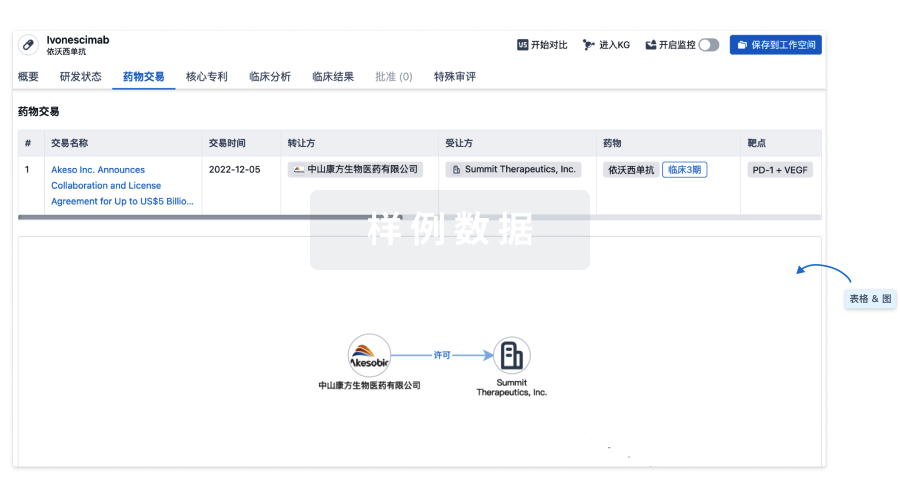
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
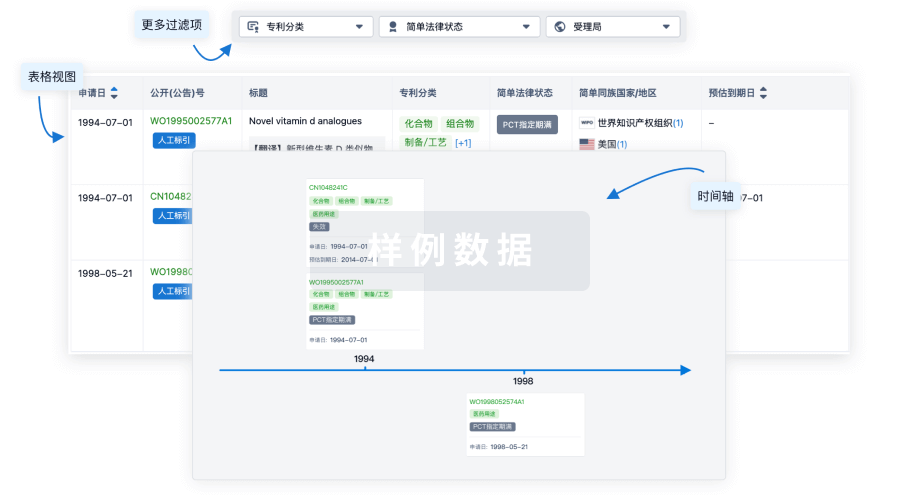
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
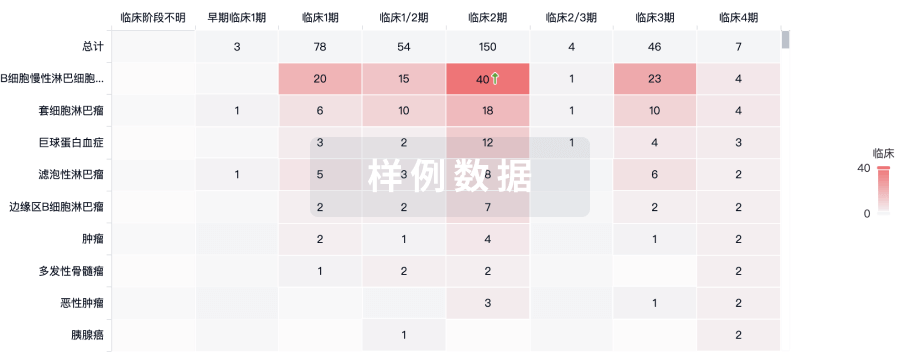
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

