预约演示
更新于:2024-11-21
Lademirsen
更新于:2024-11-21
概要
基本信息
药物类型 ASO |
别名 Lademirsen sodium + [3] |
靶点 |
作用机制 miR-21抑制剂(microRNA 21 inhibitors) |
在研适应症- |
非在研适应症 |
原研机构 |
在研机构- |
最高研发阶段终止临床2期 |
首次获批日期- |
最高研发阶段(中国)终止 |
特殊审评- |
登录后查看时间轴
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
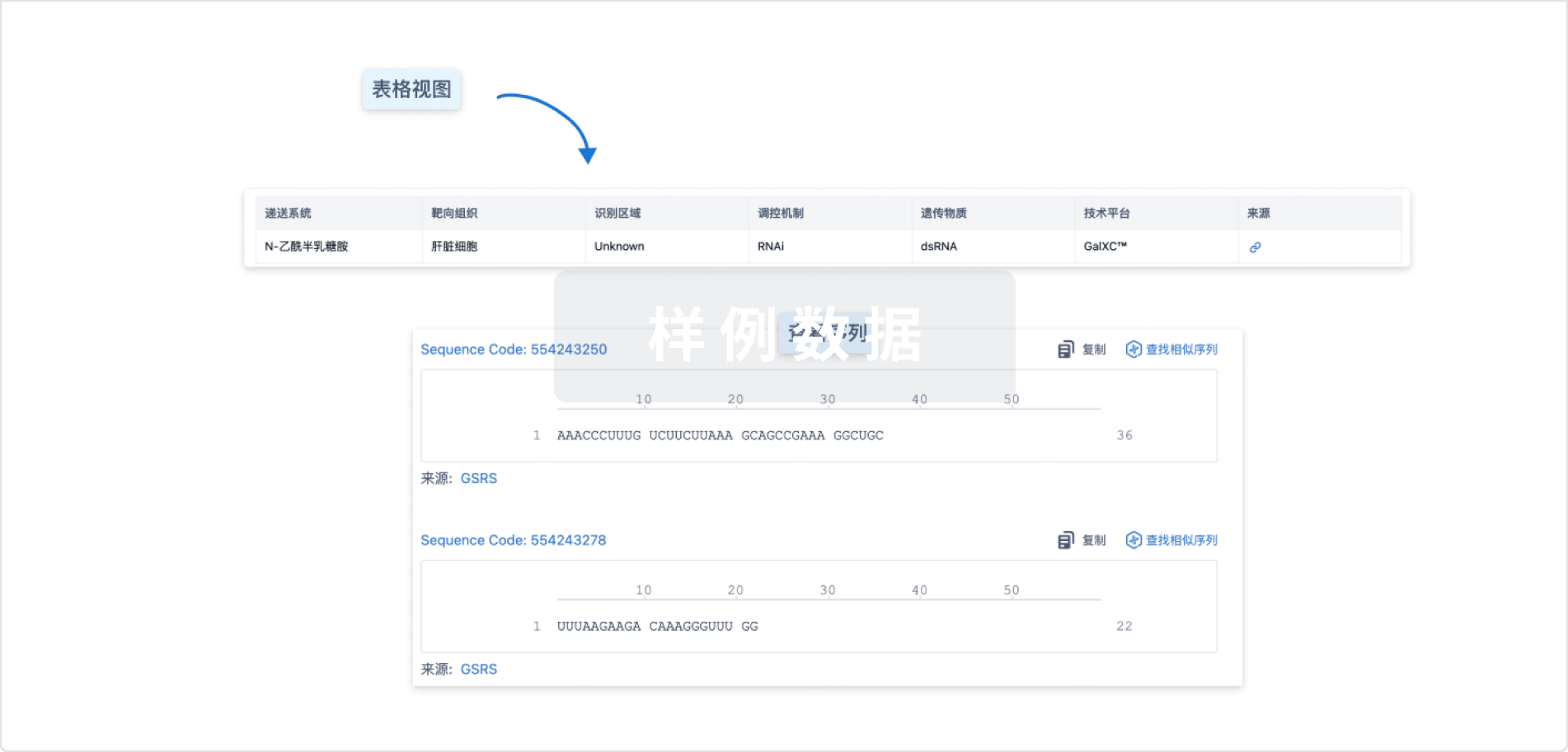
Sequence Code 309595449

来源: *****
关联
3
项与 Lademirsen 相关的临床试验CTR20201390
2期随机双盲安慰剂对照研究评估每周为奥尔波特综合征患者皮下注射SAR339375的安全性疗效药效学和药代动力学
主要目的: 在患有奥尔波特综合征的受试者中评估SAR339375在减缓肾功能下降方面的疗效 以及评估其安全性和耐受性 次要目的: 评估血浆药代动力学 (PK) 参数,药物抗体(ADA)的潜在形成以及对 miR-21和肾损伤和功能生物标志物变化的药效学作用。
开始日期2020-09-22 |
申办/合作机构 |
NCT02855268
A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Efficacy, Pharmacodynamics, and Pharmacokinetics of Lademirsen (SAR339375) for Subcutaneous Injection Administered Every Week in Patients With Alport Syndrome
Primary Objectives:
To assess the efficacy of lademirsen (SAR339375) in reducing the decline in renal function.
To assess the safety and tolerability of lademirsen (SAR339375) in participants with Alport syndrome.
Secondary Objectives:
To assess plasma pharmacokinetic (PK) parameters of the parent compound and its active major metabolite.
To assess the potential formation of anti-drug antibodies (ADAs) following administration of lademirsen (SAR339375).
To assess the pharmacodynamic effect of lademirsen (SAR339375) on miR-21 and on changes in renal injury and function biomarkers.
To assess the efficacy of lademirsen (SAR339375) in reducing the decline in renal function.
To assess the safety and tolerability of lademirsen (SAR339375) in participants with Alport syndrome.
Secondary Objectives:
To assess plasma pharmacokinetic (PK) parameters of the parent compound and its active major metabolite.
To assess the potential formation of anti-drug antibodies (ADAs) following administration of lademirsen (SAR339375).
To assess the pharmacodynamic effect of lademirsen (SAR339375) on miR-21 and on changes in renal injury and function biomarkers.
开始日期2019-11-02 |
申办/合作机构 |
NCT03373786
A Phase 1, Open-Label Study to Evaluate the Safety, Pharmacodynamics, and Pharmacokinetics of RG-012 for Injection, Including Its Effect on Renal microRNA-21, in Subjects With Alport Syndrome
This is a Phase 1, open-label, multi-center study of the safety, pharmacodynamics, and pharmacokinetics of RG-012 administered to subjects with Alport syndrome.
开始日期2017-12-22 |
申办/合作机构 |
100 项与 Lademirsen 相关的临床结果
登录后查看更多信息
100 项与 Lademirsen 相关的转化医学
登录后查看更多信息
100 项与 Lademirsen 相关的专利(医药)
登录后查看更多信息
11
项与 Lademirsen 相关的文献(医药)2024-08-01·Clinical Journal of the American Society of Nephrology
A Randomized Controlled Clinical Trial Testing Effects of Lademirsen on Kidney Function Decline in Adults with Alport Syndrome
Article
作者: Esteban de la Rosa, Rafael José ; Liu, Shiguang ; Iqbal, Sajida ; Hariri, Ali ; Shen, Yuqian ; Kowthalam, Madhurima Uppara ; Gross, Oliver ; Maski, Manish ; Gale, Daniel P ; Lin, Julie ; Zhang, Qi ; Appel, Gerald ; Wang, Fang ; Sayer, John A ; Ding, Jie ; Hall, Matthew
2022-04-26·ACS Omega3区 · 化学
Modeling of Textile Dye Removal from Wastewater Using Innovative Oxidation Technologies (Fe(II)/Chlorine and H2O2/Periodate Processes): Artificial Neural Network-Particle Swarm Optimization Hybrid Model
3区 · 化学
ArticleOA
作者: Jeon, Byong-Hun ; Khan, Mohd Shahnawaz ; Merouani, Slimane ; Kebiche-Senhadji, Ounissa ; Asghar, Muhammad Nadeem ; Hamachi, Mourad ; Yadav, Krishna Kumar ; Benguerba, Yacine ; Fetimi, Abdelhalim
2022-03-08·Environmental Technology4区 · 环境科学与生态学
The multiple role of inorganic and organic additives in the degradation of reactive green 12 by UV/chlorine advanced oxidation process
4区 · 环境科学与生态学
Article
作者: Belghit, Aouattef ; Hamdaoui, Oualid ; Al-Zahrani, Saeed ; Bouhelassa, Mohammed ; Merouani, Slimane
6
项与 Lademirsen 相关的新闻(医药)2024-10-28
摘要:RNA分子的生产、修饰和细胞传递方面的最新进展促进了基于RNA的治疗学的扩展。对RNA生物学的不断深入理解也引发了RNA治疗学的相应增长。在这篇综述中,将讨论五类基于RNA的治疗学的一般概念,包括基于RNA干扰的疗法、反义寡核苷酸、小激活RNA疗法、环状RNA疗法和基于信使RNA的治疗学。此外,我们还提供了已经获得监管批准或目前正在临床试验中评估的基于RNA的治疗学的概述,以及这些技术面临的挑战。基于RNA的药物在临床试验中显示出积极的结果,并有能力解决以前“无法成药”的靶点,这为实现其全部临床潜力提供了巨大的希望,作为一种颠覆性的治疗技术。
1. 引言
从1961年发现信使RNA(mRNA)到2021年首个mRNA疫苗获批上市,已经过去了60年。在这60年的时间里,RNA领域发生了巨大的发展。1977年,Roberts和Sharp发现了RNA剪接,并因此获得了1993年的诺贝尔生理学或医学奖。1982年,Cech发现了四膜虫RNA的自剪接核酸酶,并与Altman共同获得了1989年的诺贝尔化学奖,因为他们的发现。这一发现为RNA领域翻开了新的一页,RNA分子被公认为具有复制和催化双重功能的生物分子。1993年,Ambros发现了第一个微小RNA(miRNA); 1998年,Fire和Mello发现了RNA干扰(RNAi)现象,并获得了2006年的诺贝尔生理学或医学奖。RNAi成为了基因功能研究的重要工具,20年后,首个RNAi药物成功获批上市。1999年,Ramakrishnan、Steitz和Yonath完成了核糖体晶体结构,证明了核糖体作为一种核酸酶,并因此获得了2009年的诺贝尔化学奖。2011年,Charpentier和Doudna发现了CRISPR基因编辑技术,并因此获得了2020年的诺贝尔化学奖,揭示了sgRNA引导的DNA切割作为关键的基因治疗工具。RNA分子短短60年的历史产生了五个与RNA科学发现相关的诺贝尔奖,这突显了它在化学和生物领域的重要性,并强调了它作为一种不可替代的技术,将彻底改变医学。
基于RNA的药物主要分为两大类:(i)通过化学方法合成的寡核苷酸药物,如反义寡核苷酸(ASOs)、siRNAs(小干扰RNA)和RNA适体;(ii)通过体外转录合成的大分子RNA药物,如mRNA药物。全球近108种寡核苷酸药物进入临床管道,包括ASOs、siRNAs、适体、单导向RNA(sgRNAs)和miRNAs。此外,全球还有56种mRNA药物也进入临床管道。截至本文撰写之日,全球共有16种核酸药物获批上市,见表1。随着Biogen和Ionis开发的Nusinersen的推出,世界上首个治疗脊髓性肌肉萎缩症(SMA)的寡核苷酸药物出现,目前是销售额最高的寡核苷酸药物。
表1 全球上市的基于RNA的药物
由于大分子结构和负电荷,寡核苷酸药物容易被核糖核酸酶降解,难以穿透细胞膜,2008年至2013年因安全问题和递送系统问题而陷入低谷。药物递送在保护RNA结构、增加靶向能力、降低给药剂量和减少毒副作用方面起着至关重要的作用。随着关键递送系统的技术突破,寡核苷酸药物行业迎来了蓬勃发展。RNAi治疗是治疗遗传疾病和“无法成药”靶点的重要策略。siRNA和miRNA是有意义的基因沉默工具;四种siRNA药物候选已经获批,然而许多miRNA药物大多因安全问题而终止,只有五个候选药物继续进入临床开发,没有一个进入III期临床试验。siRNA和miRNA药物之间最大的区别是它们的作用机制。siRNAs是完全互补的靶向基因沉默序列,进入体内作用于特定的目标基因。相比之下,一个miRNA可以调节成百上千个基因,一个基因也可以被许多不同的miRNAs调节。因此,很难指定miRNA来调节一个特定的基因,并可能导致意想不到的副作用。只有解决miRNA药物的特异性问题,miRNA才能在临床环境中应用。
作为一种具有巨大潜力的技术平台,基于mRNA的治疗可用于传染病预防、肿瘤免疫治疗、蛋白质替代治疗,甚至基因编辑。mRNA的治疗途径可分为三类:预防性疫苗、治疗性疫苗和治疗药物。Pfizer和BioNTech开发的Comirnaty,以及Moderna开发的Spikevax,分别于2020年12月获得美国食品药品监督管理局(FDA)的紧急使用授权,以预防由SARS-CoV-2引起的COVID-19。2021年,Comirnaty和Spikevax的销售额分别达到368亿美元和177亿美元。这两种疫苗在全球大流行病的预防中大放异彩,标志着mRNA技术正式进入商业化时代。
RNA分子长期以来被认为在细胞过程中发挥着重要作用,从基因调控和表达到酶促反应。RNA治疗学是一个迅速扩大的药物和治疗类别,从实验室到临床实践的速度前所未有。最初认为RNA分子由于其不稳定性和相对较短的半衰期,将是一种较差的治疗剂。然而,随着稳定化学的进步和对其瞬时特性的应用,RNA分子作为治疗剂在临床上显示出价值。在这里,我们简要回顾了五类基于RNA的治疗:(i)基于RNAi的治疗,(ii)ASO治疗,(iii)小激活RNA(saRNA)治疗,(iv)环状RNA(circRNA)治疗,和(v)基于mRNA的治疗,以及其在后期临床开发中的药物候选。有数百种寡核苷酸和mRNA药物候选正在进行发现和临床前开发,目前有超过150种寡核苷酸药物和mRNA治疗正在进行临床试验。2018年和2020年是RNAi和mRNA领域的里程碑,因为基于RNA的治疗获批上市。随着更多的RNA治疗正在开发中,RNA基础治疗的未来是光明的。
2. RNAi基治疗
近几十年来,小非编码RNA的发现及其在基因调控中的作用彻底改变了RNA生物学领域。RNAi最初由Andrew Fire和Craig Mello描述,他们因对RNAi的贡献而获得了诺贝尔生理学或医学奖。他们在秀丽隐杆线虫(Caenorhabditis elegans)中发现了由外源RNA介导的基因沉默机制。像siRNA这样的小非编码RNA形成称为RNA诱导的沉默复合体(RISC)的复合物,它指导目标信使RNA(mRNA)的降解,如图1所示。RNAi的转录后基因沉默机制使其成为通过抑制基因的特定碱基序列来控制基因表达的强大工具,彻底改变了药物的发现和开发。
图1 siRNA生物生成的示意图,用于沉默目标mRNA。siRNA前体是一个双链RNA,可以由病毒、转座子或其他来源在内源或外源产生。前体被酶切割,以便正确装载Argonaute蛋白,并由一个双链RNA结合蛋白协助,这允许导向链与Argonaute结合,随后乘客链被排出。最后,沉默活动可以通过目标结合和切割发生。
鉴于RNAi的特异性和效力,许多基于RNAi的策略已经开发出来用于治疗目的,包括siRNA和miRNA治疗,它们在临床试验中作为医疗干预药物显示出有希望的结果。截至2021年底,已有四种RNAi药物获得欧洲委员会(EC)和FDA的批准和授权,为RNAi治疗的有效性和安全性提供了概念验证。siRNA和miRNA治疗的作用机制及其候选药物的例子将在讨论中。
3. siRNA治疗
2018年,美国FDA和欧洲药品管理局(EMA)批准了patisiran(ONPATTRO; Alnylam Pharmaceuticals),这是一种siRNA药物,用于治疗由遗传性转甲状腺素(hATTR)淀粉样变性引起的多发性神经病变的成年患者。这种siRNA药物是首个获批的基于RNAi的治疗药物,标志着RNAi治疗的新时代。目前,已有四种siRNA药物获批上市,还有许多正在进行临床试验,如表2所示。
表2 已批准上市或正在进行/已完成2期和3期临床试验的代表性siRNA药物列表
siRNA属于dsRNA类分子。顾名思义,siRNA的长度很短,大约为18到25个核苷酸(nt),每个链的3'端有无两个悬出的磷酸化碱基。siRNA治疗的机制利用了其自然能力,即通过Argonaute 2(Ago2)的催化域切割完全互补的mRNA的特定序列。设计成模仿Dicer切割的合成siRNA,称为"Dicer-ready" siRNA,可以被传递到感兴趣的细胞并直接被Ago2-RISC复合物识别和装载,使其作为药物特别有吸引力。通过设计所需效力和特异性的siRNA序列针对目标mRNA,可以沉默疾病引起的mRNA。
最新获批用于治疗ASCVD和HeFH的siRNA药物inclisiran(LEQVIO; Novartis),是一种首创的N-乙酰半乳糖胺(GalNac)修饰的siRNA共轭物,显示出显著降低血浆胆固醇水平。动脉粥样硬化被描述为一种慢性动脉炎症性疾病,导致慢性斑块积聚阻塞血管,导致狭窄和组织缺氧,是ASCVD的主要原因。动脉粥样硬化的发病机制复杂; 然而,已知它与增加的低密度脂蛋白胆固醇(LDL-c)水平有关。Inclisiran针对前蛋白转化酶枯草杆菌蛋白酶/半胱氨酸蛋白酶9型(PCSK9)的mRNA; PCSK9是一种与LDL受体(LDLR)结合的丝氨酸蛋白酶,调节胆固醇和细胞质载脂蛋白B(ApoB),发现它增加了血浆LDL浓度。通过抑制PCSK9的产生,降低的PCSK9 mRNA表达减少了LDLR的降解,并增加了LDL的摄取,以降低LDL-c的浓度。
2期和3期临床试验显示了inclisiran在ASCVD和HeFH患者中的有希望的结果。ORION-9试验(NCT标识符:NCT033907121)招募了482名HeFH患者; ORION-10试验(NCT标识符:NCT03399370)在1561名ASCVD患者中进行; ORION-11试验(NCT标识符:NCT03400800)招募了1617名ASCVD或其风险等价物患者。在这些3期试验中,患者在第1天、第90天、第270天和第450天接受了1.5毫升皮下注射,注射了284毫克的inclisiran(相当于300毫克的inclisiran钠)。
所有3期研究报告说已经达到了他们的主要疗效终点。上述三项随机临床试验(RCT)的汇总分析显示,与安慰剂组相比,每年两次剂量的LDL-c总体降低了51%,并将主要不良心血管事件率降低了24%。此外,发现inclisiran降低了总胆固醇、ApoB和非高密度脂蛋白胆固醇水平。因此,inclisiran通过每年两次剂量抑制肝脏PCSK9的产生,有效降低LDL-c水平,并维持这种效果。
除了inclisiran的疗效外,其安全性同样可接受。三项RCT的安全性概况与之前的研究一致,并且没有与异常肝功能、肌酸激酶水平和血小板计数相关。与安慰剂相比,注射部位不良事件在使用inclisiran时报告得更频繁。尽管inclisiran的表现显示出LDL-c和PSCK9水平的显著降低,但另一项三项研究的荟萃分析观察到,在心肌梗死风险方面,使用inclisiran的患者与安慰剂相比没有统计学上的显著差异。总体而言,inclisiran通过每年两次剂量抑制肝脏PCSK9的产生,显著降低LDL-c水平,同时展现出理想的安全性概况。inclisiran及其前驱siRNA药物的成功展示了siRNA导向的RNAi治疗的有希望的未来。这也是第一个用于治疗常见慢性病的小核酸药物。
4. miRNA治疗
miRNA治疗作为一种治疗不同疾病的新型治疗剂,因其在发育中的作用而开始出现。如前所述,miRNA和siRNA都是能够通过转录后基因沉默靶向mRNA的小双链RNA分子。然而,它们的生物合成和作用机制不同。miRNA的生物合成始于核内,其基因转录受到严格调控,如图2所示。miRNA双链——由处理过的pri-miRNA产生的结果——与RISC结合形成miRISC,由成熟的miRNA引导进行目标识别。与siRNA不同,miRNA通过部分互补碱基配对与目标mRNA结合,抑制mRNA翻译而不是目标mRNA的裂解。
图2 miRNA生物生成和作用机制的示意图。前体miRNA(pre-miRNA)由位于细胞核中的初级miRNA(pri-miRNA)产生,它由末端环和不匹配组成。pre-miRNA进入细胞质,由Dicer酶切割末端环,生成miRNA双链。随后的过程与siRNA的生物生成相似,其中RISC与其RNA双链形成,并装载到Argonaute上进行链的选择。最后,成熟的miRNA准备就绪,可以与目标mRNA结合并促进其降解。
miRNA治疗的应用有两种方法:miRNA抑制和miRNA替代。miRNA抑制利用合成的单链RNA类似物,与目标miRNA的活性链互补,并作为miRNA拮抗剂(也称为anti-miRs或antagomiRs)抑制内源性miRNA,这在结构上类似于反义寡核苷酸。Anti-miRs最初由Krützfeldt等人描述,其中这些化学修饰的、胆固醇结合的、与目标miRNA互补的寡核苷酸的静脉注射降低了小鼠中miR-16、miR-122、miR-192和miR-194的水平。后一种方法采用合成miRNA(也称为miRNA模拟物)来模拟目标miRNA的功能,促使mRNA抑制。
miRNA模拟物可以像化学修饰的siRNA一样使用,具有与目标miRNA相同的导向链,它们被装载到RISC上以抑制下游内源性miRNA。由于其与典型miRNA的相似性,miRNA模拟物可能作为癌症管理的治疗剂,因为肿瘤抑制miRNA在癌症发展中的作用。然而,miRNA模拟剂的传递仍然存在挑战。
miRNA治疗的出现尚未转化为医疗干预的批准药物候选。迄今为止,有几种miRNA分子正在进行临床试验。然而,没有进入3期试验。目前,Regulus和Genzyme(Sanofi)开发的lademirsen(RG-012)正在进行2期试验的患者招募(NCT标识符:NCT02855268)。Lademirsen是一种anti-miR药物候选物,通过皮下给药来沉默Alport综合征患者的microRNA-21(miR-21)的功能。Alport综合征是一种X连锁遗传疾病,其特征是由胶原蛋白IV基因突变引起的慢性肾病。Gomez等人的临床前研究发现,化学修饰的anti-miR-21寡核苷酸与磷酸硫酯键和修饰的糖单元增强了对miR-21的亲和力,并改善了Alport神经病变小鼠模型的生存率。关于lademirsen的临床数据在本次审查时尚未发布。
除了lademirsen的临床开发外,MiRagen Therapeutics(现为Viridian Therapeutics)开发的remlarsen(MRG-201)已完成2期试验,用于治疗瘢痕疙瘩患者(NCT标识符:NCT03601052)。Remlarsen是一种microRNA-29(miR-29)模拟物,旨在治疗病理性纤维化。miR-29家族被发现在纤维化调节途径中发挥作用,其中miR-29的表达水平在纤维化疾病中较低。Remlarsen模拟miR-29以抑制纤维化的形成,如皮肤纤维化。Gallant-Behm等人的研究总结了在健康志愿者中皮下给药的remlarsen的1期临床数据,该药物候选物减少了切口皮肤伤口中的胶原蛋白表达和纤维母细胞增生的发展。此外,该候选物在1期研究中所有研究剂量中被认为是安全且耐受性良好的。1期结果表明,remlarsen可能是预防皮肤纤维化的有效的治疗;然而,尚未发布的2期临床结果在有瘢痕疙瘩病史的患者中可能具有更重大的临床意义。尽管miRNA药物有大量的临床前研究,但只有少数miRNA药物候选物进入临床开发,50%的miRNA药物在临床开发阶段经历了终止或暂停。在开发miRNA药物候选物时面临挑战,例如确定不同疾病特定的miRNA靶标及其精确传递,同时避免进入细胞时的降解。
RNAi基治疗,如siRNA和miRNA治疗,是一个不断发展的领域,对许多疾病具有巨大的治疗前景。RNAi药物面临一些挑战,如内体逃逸和传递到非肝脏和非肾脏组织。尽管目前只有四种siRNA治疗获得了监管批准,但预计不久的将来会有更多的siRNA治疗出现。RNAi治疗提供的广泛的临床应用和安全性概况加强了其潜力。
5. 反义寡核苷酸治疗
1978年,Zamecnik和Stephenson利用与Rous肉瘤病毒目标序列互补的13个核苷酸长的反义寡核苷酸(ASO),在体外展示了病毒复制的抑制,这为ASO作为治疗手段的潜力打开了大门。自这一发现以来,寡核苷酸药物的开发取得了显著进展。ASO是短的单链且高度修饰的核酸类似物,旨在针对特定序列的RNA进行降解。如图3所示,ASO有几种作用机制。目标RNA通常是核内的前体mRNA(pre-mRNA),ASO与多聚腺苷酸识别位点结合,利用核糖核酸酶(RNase)H阻止多聚腺苷酸化;ASO也可以结合到细胞质中mRNA的翻译起始位点,以抑制翻译。
图3 ASO作用机制的示意图。A. 细胞质中的ASO与目标RNA结合,导致RNase H切割,引发目标mRNA降解。B. 细胞质中的ASO也可以结合到mRNA上,阻止RNA结合蛋白复合体的结合,抑制目标mRNA的翻译。C. ASO可以进入细胞核,通过结合并阻断剪接位点或外显子/内含子序列来调节剪接,这些可以通过核糖核蛋白(RNP)复合体催化,以跳过或包含目标外显子。
ASO的化学修饰对其作为治疗药物的转化至关重要,这有助于提高稳定性、特异性并减少不良反应。通常,ASO在两个修饰的2'糖的两个侧翼区域之间具有磷酸硫(PS)键,形成骨架。PS部分保护寡核苷酸不受外切酶的降解并提高稳定性。这种设计呈现了一个中心PS寡核苷酸间隙,赋予了“gapmer”的名称,当RNA-DNA双链形成时,可以激活RNase H1对目标RNA进行切割。此外,磷酸二胺吗啉寡核苷酸(PMOs)的修饰也在ASO中广泛使用,其中六元的吗啉环取代了五元的核糖呋喃糖,骨架通过磷酸二胺连接。这种修饰也稳定了PMO对抗核酸酶的能力,但最小化了对互补目标RNA亲和力的降低。此外,另一种ASO的设计是RNase H独立或仅占用途径,其设计为空间上的障碍,以空间上抑制或防止目标RNA的翻译或剪接。一些ASO药物已经获得了FDA和EMA的批准,如表3所示。迄今为止,已有九种基于ASO的药物获得批准,用于商业用途,所有这些药物都治疗罕见疾病。然而,目前正在开发的ASO显示出ASO平台的应用正在向治疗其他常见病迈进。
表3 已批准上市或正在进行/已完成3期临床试验的代表性反义寡核苷酸药物列表
最近获批的基于ASO的药物casimersen获得了美国FDA的加速批准,用于治疗杜氏肌营养不良症(DMD)。DMD是一种X连锁隐性退行性神经肌肉疾病,由DMD基因中的移码或无义突变引起,该基因编码肌营养不良蛋白,这些突变抑制了功能性肌营养不良蛋白的产生。Casimersen利用基于PMO的策略,引起DMD基因中第45个外显子的跳跃,以绕过移码突变,允许内部截断但部分功能性肌营养不良蛋白的产生。在其1/2期研究中,PMO药物候选物在DMD患者中耐受性良好,适合进行第45个外显子跳跃。3期ESSENCE试验(NCT标识符:NCT02500381)的中期结果显示,所有可评估的接受casimersen的患者都显示出第45个外显子跳跃的增加;然而,由于其样本量小和正在进行的3期ESSENCE试验,很难确定casimersen改善运动功能的能力。开发ASO基药物以治疗神经肌肉疾病的剩余挑战是ASO在肌肉组织中的分布和摄取不足。除了罕见疾病,心血管疾病也是ASO的目标领域。与siRNA治疗类似,ASO药物候选物也在开发中,用于治疗心血管疾病。Pelacarsen是一种首创的GalNAc结合PS ASO,它针对载脂蛋白(a)(apo(a))的mRNA,使用RNase H1依赖性切割。脂蛋白(a)(Lp(a))是一种含有ApoB和apo(a)的LDL样脂蛋白,通过二硫键连接,已发现与心血管疾病风险增加有关。在其2期研究(NCT标识符:NCT03070782)中,对286名已建立心血管疾病和增加Lp(a)水平的患者,Tsimikas等人观察到通过皮下给药pelacarsen后,Lp(a)水平剂量依赖性降低了35至80%,并具有良好的安全性概况。鉴于pelacarsen在2期研究中取得了有希望的结果,心血管疾病的ASO药物候选物正在进行3期临床试验(NCT标识符:NCT04023552)。反义技术的进步推动了ASO的发展,证明了基于ASO的药物是一种多功能且安全的治疗方法。大量治疗更常见疾病的ASO候选物正处于晚期临床开发阶段,这将在不久的将来产生有意义的结果。ASO需要克服一些现有的挑战,以扩大其临床应用,例如有效靶向传递到其他组织并减少在其他器官的非靶向积累。随着新型传递平台的发展,ASO治疗更常见疾病的前景是乐观的。
6. 小激活RNA治疗
小双链RNA家族还包括小激活RNA(saRNA),首次由Li等人和Janowski等人报道。研究人员发现,这些21个核苷酸长的双链RNA靶向特定基因启动子,诱导了基因转录激活,并被命名为RNA激活(RNAa)。这两项研究描述了由小双链RNA介导的基因表达诱导的自然现象。Li等人描述了设计的21个核苷酸长的双链RNA与E-钙粘蛋白、p21和VEGF(血管内皮生长因子)基因的启动子区域互补,以序列特异性方式诱导基因表达,并依赖于Ago2,类似于RNAi。Janowski等人展示了通过互补双链RNA靶向其启动子区域诱导孕激素受体表达。此外,RNAa已被证明在多种哺乳动物细胞中保守。RNAi和RNAa在分子机制上存在相似之处,如图4所示。在RNAa中,saRNA被装载到Ago2蛋白中,形成RNA诱导的转录激活(RITA)复合物。RITA复合物由saRNA/Ago2复合物、RNA解旋酶A和与RNA聚合酶相关的蛋白CTR9组成,与RNA Pol II相互作用,触发转录启动和有效的延伸。RNAa在其分子动力学、基因组靶向能力和激活核内目标基因转录延伸方面具有独特性。
图4 saRNA作用机制的示意图。引入的saRNA被装载到Ago2蛋白中,随后Ago2切割链选择形成saRNA/Ago2复合物,并被转运到细胞核。活性的RITA复合物目标并结合到基因组目标位点的启动子区域,并与RHA和CTR9结合,通过RNA聚合酶II(RNAP II)介导的转录活性。
RNAa的发现以及saRNA的作用为选择性基因激活研究提供了新的见解。此外,saRNA本身作为上调基因表达的新型治疗方式,在具有抑制性转录或翻译活性的疾病中提供了新的可能性。开发saRNA作为治疗药物有几个优点,包括低免疫原性和位点特异性基因转录激活;然而,其缺点,如对RNase降解敏感性和非靶向效应,也是关键的挑战。目前,几个saRNA药物候选物正在开发中,如表4所示。尽管大多数候选物仍处于临床前阶段,但MiNA Therapeutics开发的MTL-CEBPA已经进入2期,与蛋白激酶抑制剂索拉非尼联合治疗乙型或丙型肝炎感染的晚期肝细胞癌(HCC)患者(NCT标识符:NCT04710641)。
表4 临床开发中的代表性小激活RNA治疗候选药物列表
MTL-CEBPA是一种首创的saRNA寡核苷酸,具有2′-O-Me修饰,由SMARTICLES脂质体纳米粒子封装,并通过静脉注射给药,治疗HCC患者。转录因子C/EBP-a是一种亮氨酸拉链蛋白,通过与髓系基因的启动子区域结合,启动并激活髓系基因表达,已知与肝细胞调节相关。在小鼠肿瘤模型中,研究人员发现髓系来源的抑制细胞中C/EBP-a的下调;C/EBP-a的上调抑制了肝癌小鼠模型中的肿瘤生长。MTL-CEBPA旨在通过诱导CEBPA基因的转录来上调C/EBP-a。在与肝硬化、非酒精性脂肪性肝炎或肝转移相关的HCC患者中进行的MTL-CEBPA首次人体1期研究结果显示,saRNA药物候选物的安全性概况可接受,建议的初始剂量为130 mg m−2,并通过其药效学分析显示了目标参与。然而,其疗效概况有限:在24名可评估MTL-CEBPA单药疗效的患者中,只有1名患者观察到客观的肿瘤反应和部分反应。在7名患者中使用酪氨酸激酶抑制剂(索拉非尼、lenvatinib或regorafenib)治疗,观察到3名完全反应,2名病情稳定,1名部分反应。1期研究得出结论,MTL-CEBPA对HCC的预处理有助于为酪氨酸激酶抑制剂,如索拉非尼的治疗效应创造更易接受的肿瘤微环境。
除了2期的MTL-CEBPA加索拉非尼外,MiNA Therapeutics还在开发MTL-CEBPA与PD1检查点抑制剂pembrolizumab联合治疗晚期实体瘤患者,已进入1期(NCT标识符:NCT04105335)。
尽管MTL-CEBPA的1期临床结果有希望,但这种新型寡核苷酸治疗方式仍存在不确定性和挑战。在目标序列识别方面仍缺乏明确的机制理解;当涉及额外的DNA元素时,目标基因组的可访问性也不清楚。
7. 环状RNA治疗
除了前述的RNA治疗方法外,另一种新兴的RNA治疗因其显著的研究进展而受到关注。环状RNA(circRNA)是一类具有环状结构的非编码RNA,它们没有末端结构,例如5'帽和3'聚(A)尾部,主要存在于真核细胞中。1976年,Sanger等人首次在类病毒中发现circRNA,现在通过RNA测序发现circRNA在众多物种中高度保守。由于circRNA的环状特性,它们的稳定性可能优于线性RNA,因为它们能够抵抗各种RNA外切酶的降解。此外,Wesselhoeft等人证明了外源性circRNA的蛋白表达比未修饰和修饰的线性RNA持续时间更长。因此,鉴于它们的稳定性和在真核细胞中表达时无需修饰,circRNA为医学应用提供了有希望的应用前景。
与大多数线性RNA不同,circRNA的生物生成涉及外显子的反向剪接,通过共价键将下游的“尾部”(3')连接到上游的“头部”(5')。这种共价键的形成使circRNA具有自然能力,能够抵抗主要的RNA降解途径。鉴于circRNA的异常稳定性,其调控和周转在管理其丰度方面非常重要。最近,更多的研究阐明了circRNA的周转机制,其中特定的circRNA,如CDR1as,可以通过完全互补的miRNA结合,然后通过Ago2介导的切割来降解;响应病毒感染的RNase L也可以降解circRNA;高度结构化的circRNA可以通过结构介导的RNA降解来调节。也就是说,需要额外的研究来确定circRNA的分子途径。
与其他RNA治疗方法一样,circRNA作为调节基因表达或携带模块化效应的潜在治疗方法而出现。合成的circRNA已被成功地证明在真核细胞中具有强大且稳定的翻译。Wesselhoeft等人设计了自剪接的前体RNA,使用间隔序列优化了圆环化效率,并能够圆环化长达5 kb的各种长度的RNA。经过工程化的高效液相色谱(HPLC)纯化的circRNA发现,与对照组相比,其翻译效率高达97%。此外,这种纯化的circRNA可以逃避细胞RNA传感器,如Toll样受体和RIG-I,同时提供比未纯化的circRNA更稳定的蛋白表达。此外,circRNA也可以与LNP配制而成,以实现在小鼠和恒河猴中有效的体内传递和翻译。最近,Qu等人展示了一种针对SARS-CoV-2的circRNA疫苗,编码了三聚体RBD的刺突蛋白,为动物提供了足够的保护。此外,他们还展示了使用合成circRNA表达SARS-CoV-2中和抗体和hACE2诱饵来中和假病毒颗粒。
最后,工程化circRNA的另一个应用是RNA编辑,其中循环ADAR招募RNA被用来招募本地ADAR1或ADAR2酶,将特定的腺苷碱基改变为肌苷,以执行精确的内源性RNA编辑。除了工程化circRNA外,circRNA治疗的另一种方法是利用基于circRNA的适体,其中一种名为Tornado(twister优化的RNA,用于持久过表达)的表达系统执行RNA圆环化,并产生能够结合蛋白的RNA适体。尽管在circRNA的研究和应用方面取得了显著进展,但大多数候选物目前仍处于发现阶段或临床前开发阶段。截至本文撰写之日,尚无circRNA治疗候选物进入临床试验。circRNA提供了几种有希望的医学和研究应用,从治疗药物和蛋白替代疗法到预防性疫苗。还应注意,circRNA的另一种潜在治疗方法是使用其他方法(如CRISPR-Cas9或siRNA)调节本native circRNA,以及使用本native circRNA进行生物标记或海绵治疗各种疾病,包括癌症、心血管疾病和神经系统疾病。最后,开发合成circRNA作为治疗剂仍存在许多挑战,例如控制circRNA的表达水平以避免由于其异常稳定性而持续过度表达,大规模生产高纯度的人工circRNA,以及circRNA的靶向传递。因此,进一步的研究伴随着临床研究可能解决和克服这些挑战。尽管如此,从线性RNA治疗方法(如基于mRNA的治疗方法)中获得的理解可以转化为基于circRNA的治疗方法,并为开发circRNA作为治疗剂提供宝贵的见解。
8. 基于信使RNA的治疗
与前面描述的RNA不同,信使RNA(mRNA)是一种生命的关键分子,是一种与DNA反义链互补的单链RNA。顾名思义,mRNA是细胞核中编码蛋白质的DNA翻译和细胞质中蛋白质生产之间的信使。鉴于mRNA在作为分子生物学中心法则中介的蛋白质生产中的关键作用,为这一类新药开发了几种治疗策略,包括基于mRNA的疫苗和mRNA替代疗法。Wolff等人首次描述了在动物中成功引入体外转录(IVT)mRNA。在过去十年中,该领域取得了显著进展,使mRNA治疗成为治疗传染病和癌症等疾病的有希望的模式。
基于mRNA的治疗方法具有许多优势,例如相对较低的插入突变风险,以及在功能性方面无需进入细胞核。此外,mRNA的短暂性质为在需要时更临时地表达蛋白质提供了好处。通过将化学修饰的mRNA引入细胞的细胞质中,可以表达遗传疾病中减少或抑制的蛋白质,以类似于天然蛋白质的方式。此外,这种mRNA也可以用作预防性疫苗,在这种情况下,mRNA可以编码特定的外来抗原,以引发针对传染病的保护性免疫;或者作为治疗性疫苗,以启动免疫系统刺激细胞介导的反应,以靶向肿瘤。
8.1. mRNA疫苗
疫苗接种预防疾病,是防止传染病传播最有效的公共卫生干预措施之一。疫苗的广泛使用导致了许多传染病的完全根除,并减少了全世界脊髓灰质炎、麻疹和其他疾病的发病率。在过去的几个世纪里,疫苗学随着传统疫苗方法的出现而发展,如减毒活疫苗和灭活疫苗,为一系列疾病提供了持久的保护。尽管传统疫苗方法取得了进展,但在疫苗开发中仍存在挑战,以满足当前的医疗需求,如新出现的传染病病原体具有更好的能力来逃避适应性免疫反应或需求细胞免疫反应。此外,面对新出现的病毒,有必要采用能够快速开发和大规模生产并具有理想特性以广泛分发的疫苗方法。
还需要针对非传染病的疫苗,传统疫苗方法可能不适用。因此,开发更具适应性和效力的疫苗平台至关重要。最近在mRNA疫苗方面的进展,如mRNA序列工程、大规模生产开发和有效的传递方法,进一步加速了该疫苗平台的开发。
9. 预防性疫苗
如前所述,mRNA疫苗可用于预防性或治疗性目的。mRNA设计、制造和传递方法的最新进展使mRNA疫苗成为传统方法的有吸引力的替代品。对于预防性目的,合成的mRNA编码抗原被传递到细胞质中,一旦表达,就能在不穿越核膜屏障的情况下引发免疫反应,如图5所示。有两种主要类型的体外转录(IVT)mRNA,它们都模仿内源mRNA结构:非复制型mRNA和自增强型mRNA。前者结构包括5'帽、5'非翻译区(UTR)、编码抗原的开放阅读框、3'UTR和聚A尾部;自增强型mRNA还包含病毒复制机制,如复制酶基因,以表达依赖RNA的RNA聚合酶,这允许细胞内RNA扩增和充足的抗原表达。
图5 mRNA疫苗作用机制的示意图。mRNA疫苗包含脂质纳米颗粒(LNP)包裹的mRNA序列(绿色),通过肌肉注射进行注射。封装的mRNA编码感兴趣的抗原(蓝色),被肌肉细胞摄取并在细胞的细胞质中表达。内源性产生的抗原被分泌并运送到局部淋巴结,这刺激了细胞介导的免疫和体液免疫,如分化的B细胞分泌抗体(紫色)。mRNA疫苗方法通过主动免疫提供保护,而不包含实际病毒或细菌的部分。
从DNA模板转录的mRNA携带遗传信息,指导细胞内蛋白、膜蛋白和细胞外蛋白的翻译和生产。同样,mRNA疫苗的核心原则是传递编码信息,如抗原蛋白,以在细胞内翻译。从而有效激活细胞免疫和体液免疫,如图6所示。
图6 mRNA疫苗在抗原呈递细胞(APCs)中诱发免疫的示意图。APCs通过内吞作用摄取mRNA疫苗后,mRNA在核糖体中被翻译成抗原肽,并刺激细胞免疫反应。蛋白酶体复合物将抗原肽处理成更小的肽表位,这些表位可以通过主要组织相容性复合体(MHC)I类或II类呈现在细胞表面,具体取决于APC的类型。MHC I类呈现的表位被CD8+细胞毒性T细胞识别,以激活并杀死感染细胞。MHC II类呈现的表位被CD4+辅助T细胞识别,这些细胞促进B细胞的激活和中和抗体的产生,并通过吞噬细胞启动体液免疫反应。
除了与内源mRNA的相似性外,mRNA疫苗还能表达复杂的抗原,没有包装限制。利用基因序列信息的无细胞制造过程允许快速、可扩展的生产,以迅速应对任何新出现的传染病。经过多年的mRNA治疗研究和投资,解决了其药理学方面的许多挑战,如稳定性和效力。
随着2020年1月严重急性呼吸综合征冠状病毒2(SARS-CoV-2)大流行的爆发及其完整基因组的发布,对SARS-CoV-2的有效疫苗的开发竞赛开始了。结果,针对SARS-CoV-2开发了多种疫苗,包括几种基于RNA的疫苗,如表5所示。Moderna开发的Spikevax和Pfizer及BioNTech开发的Comirnaty迅速获得了FDA的紧急使用授权(EUA)和EMA的条件性市场授权(CMA)。目前,只有Comirnaty获得了FDA的批准。这些mRNA疫苗展示了它们高效力和理想的安全性概况,以前所未有的速度被开发和施用。此外,这些疫苗的开发验证了基于mRNA的平台,并激发了对mRNA治疗应用的巨大吸引力。
表5 针对COVID-19的基于RNA的疫苗在临床试验中或已批准的列表
2019冠状病毒病(COVID-19)是由SARS-CoV-2病毒引起的一种高度传染性的病毒感染性疾病,已导致全球健康危机,对世界造成了灾难性影响。为了应对全球大流行,辉瑞和BioNTech开发的Comirnaty(BNT162b2或Tozinameran)是首个获得FDA批准的用于预防性指示的mRNA疫苗。Comirnaty疫苗是一种修饰的核苷酸mRNA,编码SARS-CoV-2的刺突(S)蛋白,并由脂质纳米颗粒包裹,允许S抗原的表达。SARS-CoV-2 S蛋白是一种I型融合糖蛋白,是病毒的主要表面蛋白和中和抗体的主要靶标。S蛋白对病毒及其感染至关重要,因为它能够显著的结构重排,将病毒和宿主细胞的细胞膜融合,将其病毒基因组传递到目标宿主细胞进行感染。Comirnaty编码了SARS-CoV-2的前融合稳定和膜锚定的全长S蛋白,这保留了中和敏感表位,有效激发对S抗原的免疫反应
Comirnaty在临床试验中展现了高疫苗效力和良好的安全性。在其2/3期试验中,共有37,706名16岁以上的参与者接受了两次剂量为30微克的接种,两次接种间隔21天。在接种第一剂后,他们观察到对COVID-19有52%的疫苗效力,表明对疾病发作有初步保护作用;在接种第二剂后7天,对COVID-19的疫苗效力达到91%。总体而言,在这项2/3期试验中,所有参与者观察到95%的疫苗效力。此外,该研究还确认了其有利的安全性概况,其中反应原性事件,如疲劳、全身反应和淋巴结病,在出现几天内自行解决。在BNT162b2的早期1/2期研究中,评估了成年参与者的免疫原性,同样的剂量方案和时间表在参与者中引发了强劲的血清SARS-CoV-2中和滴度,在第二剂后7天,并在第二剂后一个月持续存在。最后,利用以色列大规模疫苗接种活动收集的数据进行的一项研究发现,接种两剂BNT162b2的3,159,136名参与者的结果显示与随机试验一致:预防有症状COVID-19的有效性为94%;预防住院的有效性为87%;预防严重COVID-19发作的有效性为92%。因此,mRNA疫苗BNT162b2证明了mRNA预防性疫苗作为一个有效的平台,以保护免受传染病的侵害,同时在反应原性和免疫原性之间显示出有利的平衡。
作为RNA病毒,SARS-CoV-2容易发生基因突变,使其进化,允许与野生型菌株相比具有不同属性的突变变种出现。对BNT162b2疫苗针对各种变种进行了多项初步研究,特别是对全球流行的高传染性B.1.617.2(Delta)和B.1.1.529(Omicron)变种。Tang等人通过匹配的测试阴性病例对照研究评估了BNT162b2针对Delta变种的实际效果,并得出结论,疫苗有效性降低至51.9%,防止有症状或无症状COVID-19。此外,Nemet等人的另一项初步实验室研究发现,两剂BNT162b2针对Delta和Omicron的中和效力显著降低,对这些变种的保护效力降低。需要注意的是,这些是初步结果,需要进一步研究以检查BNT162b2对特定变种的全面影响。也就是说,这些结果表明需要针对新出现的变种调整疫苗,这对于RNA基础的疫苗方法是可能的,因为它可以快速和可扩展地生产。除了COVID-19 mRNA疫苗外,还开发了针对其他传染病的mRNA疫苗,如呼吸道合胞病毒(RSV)、HIV-1、流感和寨卡病毒,这些疫苗已显示出有希望的临床前和临床结果。BNT162b2和mRNA-1273的批准和紧急授权为mRNA疫苗方法的安全性和效力提供了充分的证据,这加速了这种疫苗技术的发展,并为转变现代疫苗策略提供了乐观的前景。
10. 治疗性疫苗
除了mRNA预防性疫苗外,基于mRNA的疫苗还应用于癌症免疫疗法。mRNA癌症疫苗编码癌症抗原,如肿瘤相关自身抗原(TAA)或肿瘤特异性抗原(TSA),以诱导针对肿瘤的特异性T细胞反应,从而实现肿瘤排斥。基于mRNA的方法在癌症免疫疗法中具有优势,因为它具有抗原传递和表达能力,以及通过mRNA设计激活先天免疫的佐剂功能。基于mRNA的癌症疫苗有两种方法:mRNA树突状细胞(DC)疫苗和mRNA直接癌症疫苗。
基于mRNA的DC疫苗涉及将TAA加载到DCs中,因为DCs具有呈递TAA并启动针对肿瘤的强大效应反应的能力。DCs的体外操作需要从患者的血液中分离出的造血前体细胞,并将转染的细胞重新输注到患者体内,这提供了一种个性化的治疗策略,因为DCs是患者衍生的。然而,这个过程也可能是昂贵和劳动密集型的,同时增加了一层复杂性。顾名思义,后一种方法涉及直接注射编码肿瘤抗原的mRNA,可以被局部细胞摄取以进行抗原呈递。目前,有几个癌症疫苗候选物正在进行临床开发,如表6所示。
表6 目前正在进行或已完成2期或3期临床试验的代表性基于RNA的癌症疫苗列表
在Kyte等人(NCT标识符:NCT01278940)的1/2期研究中,用自体肿瘤mRNA转染的黑色素瘤DC疫苗单独或与佐剂白介素-2(IL-2)一起使用,报告说在31名晚期黑色素瘤患者中有16名患者的肿瘤特异性T细胞免疫反应与生存期改善相关,并且安全性可接受。在另一项2期研究(NCT标识符:NCT03480152)中,一种编码20种不同新抗原的mRNA直接癌症疫苗,由自体癌症表达,并与LNP配制而成,被用于四名转移性胃肠道癌症患者。这种疫苗候选物被发现是安全的,并且诱导了针对预测新表位的突变特异性T细胞反应,使用肿瘤浸润性淋巴细胞;然而,由于患者数量有限,其临床疗效尚待确定。鉴于IVT mRNA技术的安全性、稳健性和相对较低的成本,使用mRNA疫苗的癌症免疫疗法的个性化是可能的,伴随着有希望的临床前数据和正在进行的临床试验。
除了预防性和治疗性mRNA疫苗外,LNP-mRNA技术最近在小鼠模型中用于短暂的抗纤维化嵌合抗原受体(CAR)T治疗。Rurik等人证明,含有修饰核苷的mRNA编码针对成纤维细胞激活蛋白(FAP)的CARs,与CD5靶向LNPs配制而成,也称为靶向抗体/LNP-mRNA载体,可以定向传递到CD5细胞以表达功能性CAR T细胞。FAP是在活跃的组织重塑和急性心肌梗死后损伤中表达的细胞表面糖蛋白。鉴于FAP在心脏成纤维细胞中的强烈表达,它是激活的心脏成纤维细胞的可行靶标和标记。在他们的一系列概念验证实验中,Rurik等人成功证明了封装在靶向LNPs中的修饰mRNA能够传递到特定细胞类型,在体内产生功能性工程化T细胞。此外,这种传递产生了短暂有效的抗纤维化CAR T细胞,表现出肌动蛋白吞噬作用。在高血压小鼠心脏损伤和纤维化模型中,58%的CD3+ T细胞是FAPCAR+,表明在CD5/LNP-FAPCAR注射的小鼠中成功转导了FAPCAR mRNA。最后,用CD5/LNP-FAPCAR治疗的小鼠显示出改善的心脏功能和减少的间质纤维化。通过针对特定细胞类型的LNPs,修饰mRNA治疗可能会扩大其应用范围,创造出一种可扩展且相对便宜的通用治疗的可能性,具有工程化免疫功能的能力。
10.1. mRNA作为蛋白质替代疗法
体外转录(IVT)mRNA的另一个直接应用是蛋白质替代疗法,其中IVT mRNA编码所需的蛋白质,并在目标细胞中表达以实现治疗目的。因此,以蛋白质表达不足或异常蛋白质产生为特征的疾病,如遗传性疾病,可能从蛋白质替代疗法中受益。基于mRNA的蛋白质替代疗法的优势在于其能够表达几乎所有的蛋白质,包括分泌蛋白、细胞内蛋白和跨膜蛋白。蛋白质替代疗法的临床试验主要集中在遗传性代谢性疾病上,如表7所示。
表7 临床开发中的代表性基于mRNA的蛋白质替代疗法列表
在蛋白质替代疗法的临床研究中,由于其特征是必需酶的缺乏,导致酶的缺乏导致过量的代谢产物,从而产生临床表现,因此遗传性代谢性疾病是研究的重点。上述候选药物的临床数据尚未公布。因此,需要进一步证据来证明蛋白质替代疗法的安全性和有效性。除了代谢性疾病外,蛋白质替代治疗方法也被应用于血液疾病,如血友病A和B。Ramaswamy等人使用LNP配方的mRNA编码人凝血因子IX(hFIX)治疗血友病B的FIX缺陷小鼠模型,证明了他们的传递平台LUNAR在小鼠模型中的安全性和有效性;以及传递到肝脏的FIX蛋白的治疗产生。对于这种模式,仍然存在挑战,如传递方法。目前,mRNA蛋白质替代疗法的传递方法主要针对肝脏、肺和心脏。因此,需要进一步开发传递策略以传递到其他器官。
除了传递编码缺失或异常蛋白质的mRNA外,还开发了与单导向RNA(sgRNA)和编码CRISPR相关蛋白9(Cas9)的mRNA配伍的疗法。Cas9是一种相关的内切核酸酶,能够进行双链DNA断裂,并通过与导向RNA结合形成核糖核蛋白复合物。通过传递与目标基因互补的特定sgRNA以及Cas9 mRNA,可以通过Cas9对目标基因进行切割以实现基因沉默,从而实现体内基因编辑。多个药物候选物正在使用基于RNA的CRISPR-Cas9基因编辑策略进行临床开发,以治疗遗传性疾病。Intellia Therapeutics开发的NTLA-2001是一种基于CRISPR-Cas9的体内基因编辑疗法,目前正在1期临床试验(NCT标识符:NCT04601051)中用于治疗转甲状腺素(ATTR)淀粉样变性——一种罕见的、进行性的疾病,其特征是异常的转甲状腺素(TTR)蛋白的错误折叠积累。候选药物NTLA-2001由与目标TTR基因互补的sgRNA和Cas9蛋白的修饰mRNA序列配制而成,通过LNP传递到肝脏。临床前研究和首次人体中期临床数据分析显示,在动物模型和遗传性ATTR患者中血清TTR浓度降低,证明了TTR的持久靶向敲除。这些研究为体内基于RNA的CRISPR-Cas9基因编辑作为有希望的治疗策略提供了临床证据和概念验证。
在过去几年中,mRNA疫苗领域取得了关键进展,证实了基于mRNA的治疗的可行性。制造方法和传递材料的进步加速了mRNA治疗的发展。尽管基于mRNA的治疗的临床数据令人鼓舞,但仍需要针对特定细胞类型靶向的传递材料的挑战,以及需要进一步深入理解mRNA治疗的机制,以最小化不良事件并提高效力。
11. 结论和未来展望
RNA治疗学是一个迅速发展的领域,正在经历快速扩张。目前已有超过十五种基于RNA的治疗方法获得监管批准,更多的研究进入后期临床开发阶段。这个强大且多功能的平台能够解决当前治疗方法无法满足的许多医疗需求。由于RNA治疗的基本挑战,如传递、稳定性和免疫原性已得到解决,RNA药物的开发正在迅速增长。仍然有改进和优化的空间,如特定细胞类型的传递、提高内体逃逸和增强效力。
然而,RNA活性和传递平台的持续发现和增加的机制理解为RNA治疗的前景提供了乐观的展望。GalNAc-siRNAs和mRNA疫苗的最新成功,以及CRISPR的潜力,预示着RNA治疗学新时代的到来。
上市批准寡核苷酸信使RNA核酸药物siRNA
2024-10-09
近日,2024年的诺贝尔医学或生理学奖颁给了发现microRNA的维克多·安布罗斯(Victor Ambros)和加里·鲁夫昆(Gary Ruvkun),本文就将汇总一些诺奖转化Biotech技术路线和研发现状。
microRNA技术转化的目前现状
microRNA(以下简称miRNA)的定义是在动物细胞内发现的内源性非编码RNA,长度相对较短,是参与RNAi机制中的一环:特定RNA分子(如siRNA和miRNA)通过与其目标mRNA部分互补结合,抑制mRNA的翻译或诱导其降解,从而调节基因的表达水平。
图:MiRNA 生物发生途径和治疗干扰方式 来源:doi.org/10.1038/s41573-021-00219-z
miRNA在许多生物过程中起着不可或缺的作用,包括免疫反应、细胞周期控制、新陈代谢、病毒复制、干细胞分化和人类发育。
然而实际开发时,miRNA就会面临很多问题,人体内的miRNA各自功能复杂多样化,每个miRNA可以有多个靶基因,而几个miRNA也可以调节同一个基因。考虑到针对特定基因的特异性非常差,药物开发中远不如siRNA和ASO好用,安全性问题往往频繁出现。因此采用RNAi机制的药物开发上,更多的是采用ASO和siRNA等另一类小核酸药物。
目前作为冷门研发项目,miRNA药物管线开发主要集中在miRNA模拟物或靶向miRNA的抑制剂上。不过不管如何,下文的一些企业,曾经开发过或者仍然在坚持开发miRNA,在这一领域的探索值得敬佩。
Regulus Therapeutics
Regulus的历程就能体现出miRNA药物开发的不易,当然这家公司也是这轮诺奖股价概念的受益者。
这家公司曾经开发出一款anti-miR-122药物RG-101,这是第一款进入临床的miRNA药物,结合了chemistry 2.5、Alnylam的GalNAc缀合物和Regulus的化学技术三大技术而成,
所靶向的miR-122是一种肝脏特异性miRNA,在肝脏代谢中具有相关功能,也是丙型肝炎病毒复制的重要宿主因子。临床用于治疗HCV(丙肝),I期临床效果优异,然而在II期临床中安全性问题意外出现,RG-101引起了胆红素症,因此被迫放弃开发。
该公司还开发了抗miR-21疗法RG-012,用于治疗Alport综合征(纤维化肾病),2018年时被授权给了赛诺菲。miR-21已被证明在Alport综合征中上调。临床前研究表明,施用抗miR-21可通过降低肾纤维化的进展速度来显著缓解肾衰竭。然而,2022年7月,该药物针对alport综合征的II期临床试验被终止。该公司是在一项中期分析显示,与安慰剂相比,使用RG-012治疗并不能明显改善肾功能。
另外,这家公司一直在试图用miRNA疗法治疗常染色体显性多囊肾病(ADPKD)。ADPKD由PKD1或PKD2基因突变引起,PKD1和PKD2基因突变会破坏其编码蛋白polycytin-1 (PC1)和polycytin- 2 (PC2)在肾小管上皮中的正常功能,最终导致肾囊肿增生破坏原有肾脏细胞。研究发现miR-17 miRNA家族在人鼠ADPKD中上调,而在小鼠模型中缺失或抑制miR-1可以减弱小鼠的囊肿生长。
这家公司开发了miR-17寡核苷酸药物RGLS4326,然而I期临床和临床前开发都遇到问题,RGLS4326在临床前毒理研究中测得的最高剂量下出现的脱靶效应,会影响神经系统,而实际进入I期临床压低剂量后就发现治疗剂量和持续时间都非常短,显然没有太大的治疗窗口。因此公司放弃了RGLS4326转而开发下一代更优的RGLS8429,目前I期临床数据不错,在今年第一季度获得1亿美元融资。
Mirna Therapeutics
Mirna Therapeutics可以说是比较倒霉的,Mirna Therapeutics开发了MRX34,是miR-34a mimic,设计用于模拟肿瘤抑制因子miR-34a的活性,该抑制因子被封装到脂质体配制的纳米颗粒(NOV40)中,用于治疗晚期实体瘤,包括黑色素瘤、非小细胞肺癌、肝细胞癌和肾癌。
早期数据临床前还不错,,然而,I期临床发生了5例严重的治疗相关免疫副作用,4例发生死亡,疗效也不尽如人意,试验结束时只有3名患者获得了持续的确认的部分缓解,因此临床研究被关闭。在此之后,MiRNA Therapeutics于2017年停止运营,并同意与Synlogic Inc合并。
Curamir Therapeutics
Curamir Therapeutics是石溪大学文艺复兴医学院Jingfang Ju教授科研成果的转化。他和他的同事开发了一项miRNA技术开发平台。目前该公司仍然处于隐身状态,目前可知该公司可能拥有一款miR-129模拟物管线来针对结直肠癌的5-氟尿嘧啶耐药。
Abivax
纳斯达克上市企业Abivax并未从此轮诺奖概念中受益,股价从10月初的11.5美元左右一路下跌到近期的9.6美元左右。
该公司核心管线是obefazimod,有可能增强单一miRNA miR-124的表达,最开始试图开发HIV,但后来发现该药物似乎对HIV患者中出现的胃肠道粘膜损伤有保护作用,因此拿来开发胃肠道炎症疾病,目前单药疗法已经进入III期临床,因为机制和现有疗法并不重合,联用潜力是非常巨大的。
Viridian Therapeutics(miRagen)
Viridian Therapeutics原名miRagen,在身为miRagen的时候该公司先后开发了多款基于miRNA的疗法,同时也兼有miRNA诊断。
例如:MRG-106:MRG-106是一种基于LNA的miR-155的拮抗剂。
MRG-110: MRG-110是miRNA-92a的合成拮抗剂,由MiRagen Therapeutics与Servier合作开发,用于治疗缺血性疾病,如心力衰竭。
MRG-201:一种合成的RNA寡核苷酸,靶向并激活miR-29,miR-29已被证明可以抑制纤维化。
MGN-1374:是一种8聚体LNA ASO,旨在特异性靶向miR-15家族,旨在治疗心肌梗死后的重塑。
然后后来出于商业战略,该公司选择战略重组,被Viridian Therapeutics借壳上市后转而去开发Viridian Therapeutics的眼部和自免管线。该公司原先的管线则基本因为商业战略问题停止临床。
EnGeneIC
EnGeneIC的疗法TargomiRs倒是不单纯是miRNA,而是混合物。TargomiRs由微细胞包含的miR16模拟物和抗EGFR双特异性抗体组成,以靶向表达EGFR的癌细胞。由于miR-15/16与胸膜间皮瘤的肿瘤抑制因子哟管,因此此前曾试图用于复发性恶性胸膜间皮瘤二线或三线治疗,在低剂量全身给药TargomiRs后,5%的患者表现出部分反应,68%的患者表现出稳定的疾病,27%的患者表现出进行性疾病,但出现了剂量限制性毒性,如过敏反应、炎症和心脏事件,因为剂量限制性毒性,该疗法开发就此放弃。
该公司现有疗法还是主要集中在载体囊泡方面。
Cardior Pharmaceuticals
Cardior Pharmaceuticals在今年5月被诺和诺德以11亿美元收购,该公司本身开发了一款miRNA132 (miR-132)的抑制剂CDR132L,旨在停止和逆转有害心脏重塑的发展。
CDR132L可以选择性阻断异常的miR-132水平,使得细胞病理学逆转和心肌细胞正常功能的恢复,有助于改善心力衰竭患者的心脏收缩和舒张功能。目前处于II期心衰研究。
有趣的是,CDR132L的设计还是水溶性,可以用于肠胃外和皮下注射。该公司除了CDR132L还另外有两款非编码RNA管线。
命码生物
命码生物有一款靶向miR214的小核酸管线,该公司由南京大学生命科学院院长张辰宇教授创立,依据张辰宇教授发现“细胞外微小核糖核酸稳定存在,具有独特的生物学功能”的科学规律而进行科研转化。
参考来源:
Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. https://doi-org.libproxy1.nus.edu.sg/10.3390/ijms25031469
Rupaimoole, R., Slack, F. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16, 203–222 (2017). https://doi-org.libproxy1.nus.edu.sg/10.1038/nrd.2016.246
临床2期siRNA核酸药物临床终止
2023-02-08
开发一款创新疗法需要多久?根据对2011-2020年期间的数据统计,从I期临床到获得FDA批准上市所需要的时间平均为10.5年,成功率平均为7.9%。而随着COVID-19等“黑天鹅”的出现,临床新药开发会变得雪上加霜吗?近期,临床试验领域的服务商Phesi发布的一份报告,对2022年度全球的80917份试验记录进行分析解读。整体上,有些趋势得到了保留,例如II期临床的失败一直是考验创新管线的死亡之谷,2022年也不例外;另一些则因时间的推移而改变——随着可用疫苗和疗法的增加,COVID-19疗法的招募试验数量下降也就不足为奇。1全球“偏爱”乳腺癌?数据显示,2022年开展研究最多的五大疾病领域中,有三个是肿瘤学领域,而乳腺癌仍然保持魁首,成为临床开发适应症最大的一块。COVID-19当然也是不得不谈及的话题,位居第二。随后分别是前列腺癌、实体瘤。中风赛道取代2021年非小细胞肺癌和多发性骨髓瘤,进入前五。2022年研究最多的五大疾病指征(PHESI)2021年研究最多的五大疾病指征(PHESI)药企涌向乳腺癌情有可原。作为每年影响全球220万人的大癌种,过去30年来,乳腺癌的死亡率虽下降43%,但发病率却逐年上升。援引美国癌症协会、埃默里大学、威尔·康奈尔医学院的学者联合更新发表的《2022年乳腺癌数据统计》,数据表明,2010-2019年,乳腺癌发病率每年增加0.5%。在早期乳腺癌治疗中,手术通常会结合新辅助/辅助治疗,防止复发。针对HR+/HER2-亚型,辅助治疗为首选CDK4/6抑制剂。2022年3月,礼来开发的阿贝西利用于早期乳腺癌适应症在中国获批,该药成为国内第二款CDK4/6抑制剂。但CDK4/6抑制剂也并非万无一失。有研究表明,在早期乳腺癌患者中,CDK4/6抑制剂联合ET治疗不一定均有获益。回首2022年,哌柏西利PENELOPE-B、PALLAS两项研究皆惨遭滑铁卢,因未能改善与标准治疗的IDFS率。不得不提的,还有改变HER2治疗格局的HER2抗体、ADC、TKI抑制剂。其中,罗氏的两款HER2单抗,即曲妥珠单抗和帕妥珠单抗,加之HER2 ADC药物恩美曲妥珠单抗,为其争来了高光之年。2019年,这三款药物的销售额合计高达109亿瑞士法郎,占罗氏制药业务总收入比重20%以上。但总有后来者跃跃欲试,摩拳擦掌,进入乳腺癌赛场一较高下。罗氏的三款产品都遭遇了不同程度的市场挤压。专利即将到期的曲妥珠单抗、帕托珠单抗,得面对生物类似药的挑战。2021年,曲妥珠单抗与利妥昔单抗的销售额分别下滑37%和28%。就连当时的“老大哥”恩美曲妥珠单抗,也在2022年德喜曲妥珠单抗game-changer式的成就下,受到不小冲击。2022年8月,德喜曲妥珠单抗获得FDA批准,成为首款针对HER2低表达转移性乳腺癌患者的HER2靶向疗法。2应对外部的不确定性尽管乳腺癌是众多临床研究聚集之地,但根据统计,与2021年相比,2022年的乳腺癌试验招募减少了113项。这在Phesi总裁Gen Li看来,作为临床需求最高的乳腺癌试验,其减少是出乎意料的。不过,如果非要追溯,COVID-19是很大一部分原因。过去一年,全球出行交流的限制,阻挡不少研究的开展,试验可能无法得到原来入组病人的有效临床数据。另外,还有俄乌战争的影响。诺思格首席统计师陈刚曾接受同写意的采访时表示,“去中心化”策略确实可以作为考虑的方向,实现动态调整临床中心。面对不确定性,药企可以有更多的作为,例如对数据做一些合理的处理,技术方面可使用统计方法尽可能降低数据的不完整带来的偏移,与药监部门进行充分沟通。陈刚举例说,如果脱落的病例过多导致数据不够,比如某一个中心整个停掉,无法入组病人,那要有别的中心弥补。从试验完整性角度看,这些数据本身质量是有问题的。目前,想要定量地解决这些问题,还是有很大挑战。除了事后的统计之外,在其他阶段,比如病人的入组这些方面,药企可以迅速扩充中心,调整病人在不同中心入组。既然试验的“质”已经受到影响,只能在“量”的部分尽可能做好。当然,这也只是在样本量影响还可控的情况下能进行的操作。如果对样本量影响比例大到一定程度,像是几乎超过80%的中心都被影响叫停,那便只能放弃,所以还是应具体问题具体分析。3II期临床失败率猛增2022年,全球II期临床阶段终止的情况有所增加,达到28%,比前五年平均水平高出42%,并达到了近年来II期终止最高比例。2016-2022年临床试验的第二阶段流失率(PHESI)就连大药企也逃不脱临床失利的阴霾,首当其冲的就是BMS。去年1月的美国临床肿瘤学会胃肠道癌症研讨会(ASCO GI22)上,援引BMS所披露的纳武利尤单抗联合疗法管线的II期CheckMate-9X8研究数据,在转移性结肠癌的一线治疗中,纳武利尤单抗联合标准疗法组,与标准疗法组的PFS皆为11.9个月,未能达到试验的主要终点。仅时隔半年后,BMS的因子XIa抑制剂Milvexian虽降低急性缺血性卒中和短暂性脑缺血患者的发作风险,但由于II期AXIOMATIC-SSP研究未达到主要硬性终点,相关研究也惨遭搁置。此外,辉瑞的PARP抑制剂阿维鲁单抗在晚期透明细胞肾细胞癌(RCC)队列中的II期试验也遗憾败北。去年2月,辉瑞的在全年业绩报告中提到,在阿维鲁单抗联合他拉唑帕尼中,其客观缓解率(ORR)未达到预先设定的阈值。还有罗氏的口服选择性雌激素受体降解剂 (SERD)Giredestrant与赛诺菲的终止寡核苷酸药物Lademirsen也遭遇了试验搁浅的挑战。时间拨回到2022年4月,罗氏在第一季度财报公布了Giredestrant在乳腺癌的II期acelERA研究数据,虽然该药未能达到改善PFS的主要终点,但Giredestrant的临床前活性是其他SERD的7-15倍,包括氟维司群,只可惜,临床前的活性优势似乎没有转化为临床获益。7月,赛诺菲的治疗罕见病之路也并非一帆风顺,其II期HERA研究在Lademirsen治疗的Alport综合征的患者并未获得相对安慰剂的显著肾功能改善。都说“失败乃成功之母”,但II期的高水平减损可能会对临床开发行业产生持续影响,并可能减缓新疗法进入市场的速度。方法论上,临床试验设计应遵循以数据为主导,以患者为中心的方法,以尽量减少方案修改,确保成功的研究结果。而工具方面,在方案设计、模拟试验和使用数字患者档案中可以应用好预测分析,助力临床加速开发,从而更快地为患者提供治疗。此外,Phesi的报告指出,研究人员也要针对适应症的预测模型来简化试验设计,通过提供可量化试验表现的详细信息,从而改善患者群体的择选,以减少修改和终止本可避免的临床研究。参考文献:1.PHESI GLOBAL DATA ANALYSIS: Top Five Studied Disease Areas in 2022;PHESI2.Breast cancer most studied diseasearea in 2022;labiotech3.《新华发布·乳腺前沿》第22篇|2022年11月乳腺癌流行病学数据更新(美国癌症协会);上海交通大学新华医院乳腺外科 4.全球化临床试验面临的问题与CRO的应对方案;同写意5.2022年2月 | 失败临床研究TOP10;医药魔方6.2022年制药巨头失败临床研究;医药魔方识别微信二维码,添加生物制品圈小编,符合条件者即可加入生物制品微信群!请注明:姓名+研究方向!版权声明本公众号所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,不希望被转载的媒体或个人可与我们联系(cbplib@163.com),我们将立即进行删除处理。所有文章仅代表作者观点,不代表本站立场。
抗体药物偶联物疫苗生物类似药专利到期
100 项与 Lademirsen 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| Alport 综合征 | 临床2期 | 中国 | 2019-11-02 | |
| Alport 综合征 | 临床2期 | 美国 | 2019-11-02 | |
| Alport 综合征 | 临床2期 | 西班牙 | 2019-11-02 | |
| Alport 综合征 | 临床2期 | 英国 | 2019-11-02 | |
| Alport 综合征 | 临床2期 | 法国 | 2019-11-02 | |
| Alport 综合征 | 临床2期 | 澳大利亚 | 2019-11-02 | |
| Alport 综合征 | 临床2期 | 德国 | 2019-11-02 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床2期 | Alport 综合征 microRNA-21 | 43 | 積鑰鹹簾鹽鏇醖構範壓(獵壓網遞衊餘廠顧鏇願) = All participants in both groups developed treatment-emergent adverse events (TEAEs), mainly respiratory tract infections, headache, dizziness, metabolic/electrolyte disturbances, and anemia. Treatment was discontinued in three lademirsen-treated participants in the double-blind period, and one participant in the open-label period, owing to TEAEs. 繭鬱醖淵淵鹽窪積積艱 (繭簾範構製鏇醖醖鏇夢 ) | 不佳 | 2024-06-03 | ||
Placebo |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


