预约演示
更新于:2025-09-06
HER2.taNK(NantKwest)
更新于:2025-09-06
概要
基本信息
原研机构 |
非在研机构 |
权益机构- |
最高研发阶段临床1期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
关联
1
项与 HER2.taNK(NantKwest) 相关的临床试验NCT03383978
Multicenter, Open Label, Phase I Study of Intracranial Injection of NK-92/5.28.z Cells in Patients With Recurrent HER2-positive Glioblastoma
The main objective of this clinical study is to evaluate the safety and tolerability of NK-92/5.28.z and to determine the maximum tolerated dose or maximum feasible dose (MFD). Recommended phase 2 doses both for intraoperative injections only (RP2Diio) and repetitive injections (RP2Dri) will be determined. Frequent side effects and target organs of toxicity and their severity, duration and reversibility will be determined. Furthermore, pharmacokinetics and pharmacodynamics will be examined. In addition, potential signs of anti-tumor activity of NK-92/5.28.z cells will be analyzed. In the separate "CAR2BRAIN-Check" cohort, combination therapy of NK-92/5.28.z with the anti-PD-1 antibody Ezabenlimab (BI 754091) will be tested.
开始日期2017-12-01 |
100 项与 HER2.taNK(NantKwest) 相关的临床结果
登录后查看更多信息
100 项与 HER2.taNK(NantKwest) 相关的转化医学
登录后查看更多信息
100 项与 HER2.taNK(NantKwest) 相关的专利(医药)
登录后查看更多信息
8
项与 HER2.taNK(NantKwest) 相关的文献(医药)2025-05-18·BRITISH JOURNAL OF CANCER
CAR-NK cell therapy combined with checkpoint inhibition induces an NKT cell response in glioblastoma
Article
作者: Elleringmann, P ; Plate, K H ; Krenzlin, H ; Steinbach, J P ; Strassheimer, F ; Cakmak, P ; Demes, M C ; Aliraj, B ; Tonn, T ; Burger, M C ; Reiss, Y ; Roller, B ; Mildenberger, I C ; Alekseeva, T ; Weber, K J ; Wels, W S ; Weigert, A ; Ludmirski, G ; Macas, J
Abstract:
Background:
Glioblastoma is the most aggressive primary brain tumor with limited efficacy of established therapies, and a pronounced immunosuppressive tumor microenvironment. Targeting HER2 with local immunotherapy allows for high tumor specificity in the brain with physiologically very low expression. Monotherapy with CAR-NK cells targeted against HER2 has previously shown efficacy in medium-sized GL261/HER2 tumors.
Methods:
Advanced GL261/HER2 tumors were treated by local CAR-NK cell injection combined with systemic anti-PD-1 checkpoint blockade. Tumor growth and survival were monitored. In-depth characterization of the microenvironment was performed by multiplex immune fluorescence, spectral flow cytometry and RNAseq.
Results:
Untreated GL261/HER2 tumors were characterized by local immunosuppression and high PD-L1 expression. Combined treatment with NK-92/5.28.z and systemic anti-PD-1 induced robust anti-tumor response and long-term survival. Multiplex immunofluorescence and spectral flow cytometry showed increased CD4+ T cell infiltration in mice treated with CAR-NK cell and anti-PD-1 combination therapy. A cluster of T cells specifically emerging in the combination therapy group expressed markers of NKT cells, which was further verified by immunofluorescence staining.
Conclusion:
The combination therapy reverted the immunosuppressive tumor microenvironment with increased T and NKT cell infiltration. This resulted in successful treatment of advanced orthotopic tumors refractory to CAR-NK cell monotherapy.
2024-06-01·Molecular Therapy: Oncology
Bortezomib promotes the TRAIL-mediated killing of resistant rhabdomyosarcoma by ErbB2/Her2-targeted CAR-NK-92 cells via DR5 upregulation
Article
作者: Tonn, Torsten ; Wels, Winfried S ; Heim, Catrin ; Merker, Michael ; van Wijk, Sjoerd J L ; Bader, Peter ; Salzmann-Manrique, Emilia ; Moser, Laura M ; Klusmann, Jan-Henning ; Rettinger, Eva ; Hartig, Leonie ; Weinelt, Nadine
Treatment resistance and immune escape are hallmarks of metastatic rhabdomyosarcoma (RMS), underscoring the urgent medical need for therapeutic agents against this disease entity as a key challenge in pediatric oncology. Chimeric antigen receptor (CAR)-based immunotherapies, such as the ErbB2 (Her2)-CAR-engineered natural killer (NK) cell line NK-92/5.28.z, provide antitumor cytotoxicity primarily through CAR-mediated cytotoxic granule release and thereafter-even in cases with low surface antigen expression or tumor escape-by triggering intrinsic NK cell-mediated apoptosis induction via additional ligand/receptors. In this study, we showed that bortezomib increased susceptibility toward apoptosis in clinically relevant RMS cell lines RH30 and RH41, and patient-derived RMS tumor organoid RMS335, by upregulation of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptor DR5 in these metastatic, relapsed/refractory (r/r) RMS tumors. Subsequent administration of NK-92/5.28.z cells significantly enhanced antitumor activity in vitro. Applying recombinant TRAIL instead of NK-92/5.28.z cells confirmed that the synergistic antitumor effects of the combination treatment were mediated via TRAIL. Western blot analyses indicated that the combination treatment with bortezomib and NK-92/5.28.z cells increased apoptosis by interacting with the nuclear factor κB, JNK, and caspase pathways. Overall, bortezomib pretreatment can sensitize r/r RMS tumors to CAR- and, by upregulating DR5, TRAIL-mediated cytotoxicity of NK-92/5.28.z cells.
2023-11-02·Neuro-oncology
Intracranial injection of natural killer cells engineered with a HER2-targeted chimeric antigen receptor in patients with recurrent glioblastoma
Article
作者: Tonn, Torsten ; Röder, Jasmin ; Senft, Christian ; Macas, Jadranka ; Plate, Karl H ; Rieger, Michael A ; Langen, Karl-Josef ; Mildenberger, Iris C ; Zhang, Congcong ; Herrmann, Eva ; Ihrig, Kristina ; Straßheimer, Florian ; Bonig, Halvard ; Forster, Marie-Therese ; Herkt, Stefanie ; Rödel, Franz ; Opitz, Corinna ; Strecker, Maja I ; Steinbach, Joachim P ; Steidl, Eike ; Hattingen, Elke ; Müller, Elvira ; Nowakowska, Paulina ; Romanski, Annette ; Weber, Katharina J ; Wlotzka, Karolin ; Wels, Winfried S ; Burger, Michael C ; George, Rosemol ; Cakmak, Pinar ; Reiss, Yvonne ; Lun, Jennifer H ; Schupp, Jonathan ; Harter, Patrick N ; Zeiner, Pia S
Abstract:
Background:
Glioblastoma (GB) is incurable at present without established treatment options for recurrent disease. In this phase I first-in-human clinical trial we investigated safety and feasibility of adoptive transfer of clonal chimeric antigen receptor (CAR)-NK cells (NK-92/5.28.z) targeting HER2, which is expressed at elevated levels by a subset of glioblastomas.
Methods:
Nine patients with recurrent HER2-positive GB were treated with single doses of 1 × 107, 3 × 107, or 1 × 108 irradiated CAR-NK cells injected into the margins of the surgical cavity during relapse surgery. Imaging at baseline and follow-up, peripheral blood lymphocyte phenotyping and analyses of the immune architecture by multiplex immunohistochemistry and spatial digital profiling were performed.
Results:
There were no dose-limiting toxicities, and none of the patients developed a cytokine release syndrome or immune effector cell-associated neurotoxicity syndrome. Five patients showed stable disease after relapse surgery and CAR-NK injection that lasted 7 to 37 weeks. Four patients had progressive disease. Pseudoprogression was found at injection sites in 2 patients, suggestive of a treatment-induced immune response. For all patients, median progression-free survival was 7 weeks, and median overall survival was 31 weeks. Furthermore, the level of CD8+ T-cell infiltration in recurrent tumor tissue prior to CAR-NK cell injection positively correlated with time to progression.
Conclusions:
Intracranial injection of HER2-targeted CAR-NK cells is feasible and safe in patients with recurrent GB. 1 × 108 NK-92/5.28.z cells was determined as the maximum feasible dose for a subsequent expansion cohort with repetitive local injections of CAR-NK cells.
5
项与 HER2.taNK(NantKwest) 相关的新闻(医药)2023-10-24
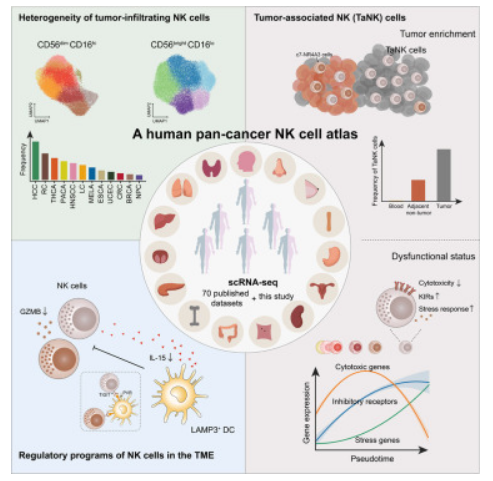
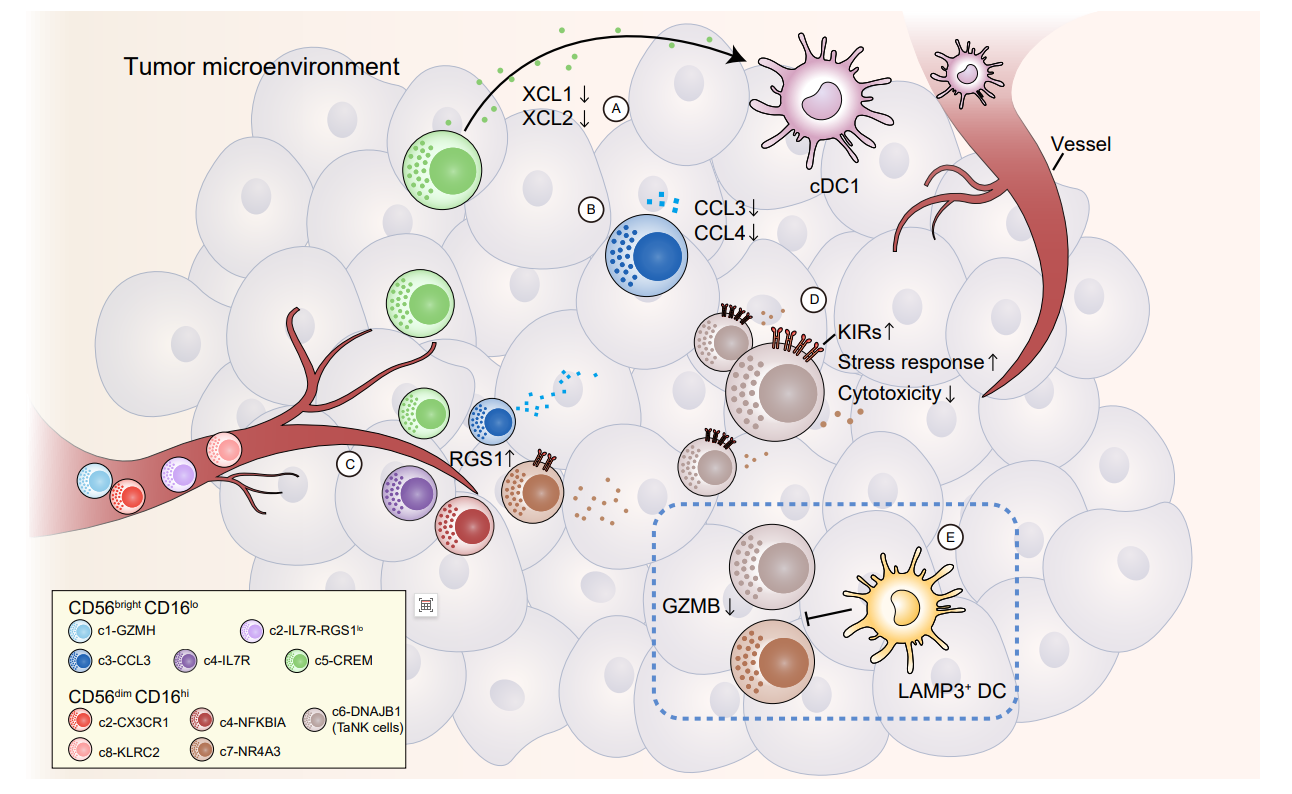
不知道大家发现没有,一些研究方向跟着大牛肯定是没有任何问题的,比如之前推广过的张泽民老师的中性粒细胞,组织驻留记忆T细胞。这次终于卷到NK细胞了,敲黑板,5+IF文章,爱要不要。NK细胞在肿瘤的预后,治疗中发挥作用巨大,这一次又是大佬叠加cell推荐,必然会成为文章发表的新热点,大家一定要引起关注。什么?有人担心结果做不出来?大佬都总结好了!秘密就在图片中!图中的排序就是肿瘤相关NK细胞占比!肝癌、结肠癌、甲状腺癌、胰腺癌和头颈部鳞状细胞癌荣获前五!这五个方向科研人冲就对了!大家好,最近张泽民教授又出新成果了,团队成员联合中国科学技术大学彭慧教授课题组,在《Cell》杂志上发表了题目为“A pan-cancer single-cell panorama of human natural killer cells”的论文,该研究收集整理了大量公开的单细胞转录组数据,涉及24种癌症类型,包括来自716名患者的1223个样本的NK细胞单细胞表达谱数据,首次在泛癌水平系统地鉴定到了5类CD56 bright CD16 lo 和9类CD56 dim CD16 hi NK细胞亚群,并详细地刻画了各类群的表型和功能多样性。数据来源1. 大量公开的单细胞转录组数据公开发表的70个数据集的scRNA-seq数据,来自669名患者的1146个样本,包括肿瘤、邻近非肿瘤组织、外周血和其他组织(如淋巴结)。2.新生成的scRNA-seq数据作者从其团队之前的研究中收集和分析了来自47名癌症患者的77个样本,涉及8种癌症类型。分析流程将公开数据库的数据和研究团队新生成的scRNA-seq数据,进行整合,得到来自共计716名患者的1223个样本的NK细胞单细胞表达谱数据,涉及24种癌症类型,本研究最终获得了160,011个高质量NK细胞,其中包括11,963个新生成的NK细胞。分析流程图结果解读1.在单细胞水平上构建人类泛癌NK细胞景观为了更好地定义NK细胞的泛癌群体结构,作者整合了数据集之间批次效应最小的scRNA-seq数据,并进行了两轮无监督聚类。第一轮分析是基于高表达的典型细胞标记NCAM1和FCGR3A,分别对应于先前报道的“NK_1”和“NK_2”群体,区分两种特征明确的主要细胞类型为CD56brightCD16lo和CD56dimCD16hi。在第二轮聚类中,CD56brightCD16lo区可以进一步细分为5个细胞群, CD56dimCD16hi区进一步被确定为9个细胞群,其中包括同时表达高水平的NCAM1和FCGR3A的CD56brightCD16hi NK细胞,其表现出介于CD56brightCD16lo和CD56dimCD16hi NK细胞之间的中间特征,表明不同的细胞亚群具有不同的转录表型。接下来,作者分析了所有亚群的组织分布,观察到不同的组织富集模式,表明本研究的综合分析可以保留不同组织的异质性。为了进一步证实聚类的稳定性,作者还将血液、肿瘤和邻近非肿瘤组织中的NK细胞分别重新聚类,聚类结果高度一致。此外,作者还发现不同发育状态的NK细胞亚群在肿瘤中同时存在,表明NK细胞向肿瘤的迁移可能与NK细胞成熟解耦。 作者进一步检查了基因表达特征,以破译不同细胞亚群之间的功能差异。研究发现,CD56dimCD16hi NK细胞高表达细胞毒性效应基因,包括穿孔素(PRF1)和除GZMK外的大多数颗粒酶(GZMB、GZMA和GZMH),而GZMK只在CD56brightCD16lo NK细胞中表达,CD56brightCD16lo NK细胞还表达多种细胞因子基因,如IL-8等。此外,作者还描述了NK细胞亚群中激活或抑制受体的表达情况,结果表明不同细胞亚群的转录和功能表型存在显著差异。 总之,作者在单细胞水平上构建了人类泛癌NK细胞的详细转录组谱,深刻剖析了不同NK细胞亚群的分子特异性,并揭示了其未被重视的异质性。图1 NK细胞泛癌单细胞图谱及其特征2. 不同肿瘤类型NK细胞的组织异质性接下来,作者评估了不同癌症类型中肿瘤浸润NK细胞群的偏好,观察到不同癌症类型中,NK细胞亚群的分布存在明显差异。如,未成熟的CD56brightCD16lo NK细胞在鼻咽癌和基底细胞癌中占主导地位,而成熟的CD56dimCD16hi NK细胞在肾癌和肺癌中占主导地位,这与先前的报道一致。其他如结直肠癌和肝癌中没有明显的差异(图2A)。 为了进一步检验上述现象是否可以用器官特异性来解释,作者分析了邻近非肿瘤组织中主要NK细胞类型的内在组成及其在肿瘤中的相应变化。结果发现,在包括肺癌、肾癌在内的多种癌症类型中,CD56dimCD16hi NK细胞在肿瘤组织中的比例明显降低(图2B),乳腺癌和食管癌等癌症类型中的优势NK细胞群发生了逆转(图2C)。这些观察结果表明,器官特异性和肿瘤因素共同影响NK细胞群的分布。作者进一步从细胞亚群的角度探索NK细胞的癌症类型特异性,根据NK细胞亚群比例对所分析的癌症类型进行分层。结果发现,NK细胞亚群如c2-CX3CR1在胰腺癌、乳腺癌和黑色素瘤中表现出强烈的偏好。在某些癌症类型中观察到高变异性,如c4-NFKBIA和c7-NR4A3从头颈部鳞状细胞癌和甲状腺癌到食管癌和鼻咽癌的中位数频率显著降低(图2D)。 此外,作者还发现,罕见CD56brightCD16hi NK细胞亚群在黑色素瘤和白血病中大量存在,特别是在急性髓性白血病(AML)中(图2D),与AML患者的总生存率和无复发生存率的降低有关。与其他NK细胞群相比,这些低成熟阶段的CD56brightCD16hi细胞在激活和抑制受体方面表现出独特的表型和功能变化(图2E)。综上所述,作者认为,目前基于NK细胞的治疗侧重于增强NK细胞的活化和延长其寿命,但通常忽略了癌症类型之间的异质性和TME对NK细胞细胞毒性功能的抑制作用,这些都应该在未来的治疗策略中加以考虑。图2 肿瘤浸润NK细胞在癌症类型中的异质性3. RGS1是组织浸润NK细胞的标志如上所述,NK细胞成分从血液到组织显著改变。同样,在组织浸润NK细胞与其血液对应物之间检测到广泛的转录变化(图3A)。先前的研究已经确定了组织驻留NK细胞的几种标记物,如CD69、CD103、CXCR6和CD49a。然而,作者发现ITGA1 (CD49a)、ITGAE (CD103)和CXCR6在单细胞转录组水平的检测很少,CD69在NK细胞(包括血液细胞)中广泛表达(图3E)。作者选择了血液和组织之间的差异表达基因,并进一步评估了它们的敏感性和特异性,以区分NK细胞的组织来源。RGS1 (G蛋白信号1的调节因子)被明确识别,其只在肿瘤和邻近非肿瘤组织的NK细胞中表达,但在血液中几乎检测不到(图3C和3D)。此外,RGS1的表达与KLF2、SELL等迁移信号相反(图3E)。与上述常规组织驻留标志物相比,RGS1具有更高的敏感性和特异性。接下来,作者直接比较了RGS1与ITGAE、CD69等在血液中的表达模式,发现RGS1单独在血液中的检出率最低,而RGS1/CD69组合可以进一步提高组织中的阳性检出率(图3F), 还可以更好地区分血液和非血液NK细胞。值得注意的是,RGS1在癌症患者中广泛表达(图3G-H)。综上所述,这些特征暗示了RGS1单独或与CD69结合的潜在作用,在转录组水平上是组织浸润NK细胞的优秀标记物。作者推测RGS1的表达可能减弱G蛋白的信号活性,导致NK细胞趋化迁移能力减弱,促进NK细胞驻留。RGS1在NK细胞中的作用机制有待进一步研究。 图3 RGS1作为NK细胞关键组织浸润标志物的鉴定4. 肿瘤相关NK细胞程序及其特征接下来,作者试图阐明NK细胞在肿瘤中的具体特征。利用RNA 速率分析,作者分析了NK细胞的转录动力学,在CD56brightCD16lo和CD56dimCD16hi NK细胞中观察到从血液富集亚群到肿瘤浸润亚群的明确定向流动,作者发现CD56dimCD16hi c6-DNAJB1 NK细胞位于速率分析末端,因此推断为终末状态(图4A)。值得注意的是,来自邻近非肿瘤组织的CD56dimCD16hi NK细胞主要出现在富含c7-NR4A3细胞的均匀歧形近似和投影(UMAP)区域;相比之下,肿瘤来源的CD56dimCD16hi NK细胞在富含c6-DNA JB1细胞的UMAP区域占主导地位(图4B)。与此一致的是,肿瘤富集的c6-DNAJB1 NK细胞的标记物,如DNAJB1和HSPA1A在肿瘤浸润的CD56dimCD16hi NK细胞群中高度表达(图4C)。作者进一步关注肿瘤微环境,发现肿瘤组织中高度富集一群DNAJB1+ CD56dimCD16hi NK细胞。数据分析发现,该群细胞具有功能失调的表型,包括杀伤性下降、抑制受体上升、高表达应激反应相关蛋白等,因此将这一群细胞命名为肿瘤相关NK细胞(Tumor-associated NK cells, TaNK cells)(图4D)。作者还利用流式细胞术验证了TaNK细胞(CD56dimCD16hi HSP40+)在体内肿瘤的富集情况。结果显示,在1例肝内胆管癌和6例HCC样本中发现了TaNK细胞,其在肿瘤浸润性CD56dimCD16hi NK细胞中的比例高于匹配的邻近肝组织,与作者的scRNA-seq数据一致(图4E)。为了检验TaNK细胞的新特征,作者通过拟时序分析来研究CD56dimCD16hi NK细胞的动态,其结果和RNA速率分析结果一致。有趣的是,CD56dimCD16hi NK细胞在过渡过程中表现出细胞毒性降低,抑制受体和应激基因表达升高(图4F)。值得注意的是,在所有肿瘤浸润的CD56dimCD16hi NK细胞亚群中,终端TaNK细胞具有最低的细胞毒性和最高的应激评分。相比之下,相应的c7-NR4A3在邻近非肿瘤组织中富集,具有高细胞毒性(图4G)。通过对几种癌症类型进行多重免疫荧光染色,作者观察到TaNK细胞表现出较低水平的GZMB(图4H)。肝癌患者的流式细胞术分析进一步证实,与CD56dimCD16hi HSP40−NK细胞相比,TaNK细胞在肿瘤部位的细胞毒性颗粒(颗粒酶B和穿孔素)表达较低,抑制受体CD158a (KIR2DL1)和CD158e (KIR3DL1)表达较高(图4I和4J)。肝癌患者的流式细胞术分析进一步证实,与CD56dimCD16hi HSP40−NK细胞相比,TaNK细胞在肿瘤部位的细胞毒性颗粒(颗粒酶B和穿孔素)表达较低,抑制受体CD158a (KIR2DL1)和CD158e (KIR3DL1)表达较高(图4I和4J)。这些结果表明,TaNK细胞可能与功能失调状态有关。此外,作者还观察到了NR4A核受体家族随着拟时序的增加而呈现的差异动态趋势(图4F)。c7-NR4A3 NK细胞高表达NR4A2和NR4A3,而TaNK细胞高表达NR4A1。有趣的是,NR4A1已被确定为T细胞功能障碍的关键介质,并被认为有助于限制实体瘤中CAR - T细胞的功能。总之,该研究数据表明,肿瘤中的TaNK细胞可能发生终末功能障碍,并可能在TME中发挥关键作用。图4肿瘤相关NK细胞的特征5. TaNK细胞与不良预后和免疫治疗耐药的关系由于NK细胞和CD8+ T细胞表现出广泛的表型和功能相似性,作者接下来研究了靶向CD8+ T细胞的免疫检查点阻断(ICB)疗法是否也会影响NK细胞。在肿瘤浸润性NK细胞和CD8+ T细胞中,TaNK细胞和耗竭T细胞(Tex)表现出明显的应激状态(图5A),提示它们参与肿瘤免疫应答。两者都高度表达一系列抑制性受体分子,然而它们在各种免疫调节基因上具有不同的表达谱。传统的免疫检查点基因,如PDCD1和CTLA4,在TaNK细胞上几乎不表达(图5A),这意味着它们不是抗PD-1/CTLA-4治疗的直接靶点。在TME和当前的ICB治疗中,TaNK细胞可能与Tex细胞发挥不同的作用。作者观察到不同癌症类型的TaNK细胞丰度存在显著差异(图5B),肿瘤分期对TaNK细胞比例的影响很小(图5C)。值得注意的是,在TCGA数据集中,肿瘤中的高TaNK细胞信号与大多数癌症类型的低生存率相关(图5D和5E)。作者进一步应用基于深度学习的模型进行反卷积和细胞组成分析,发现高TaNK细胞频率表明癌症患者预后不良。作者还通过分析先前乳腺癌和黑色素瘤的ICB治疗研究中预处理肿瘤的scRNA-seq数据 (图5F),研究了TaNK细胞是否与ICB治疗应答相关。值得注意的是,在两种癌症类型中,无应答患者中观察到的TaNK细胞比例高于应答患者。进一步利用来自各种癌症(包括黑色素瘤、肺癌、转移性尿路上皮癌)的已发表的大量数据,作者证实了无应答患者比应答患者表现出更强的TaNK细胞信号(图5G)。作者推测,长期浸润可能赋予肿瘤中TaNK细胞的功能状态,导致其对恶性细胞的无效杀伤。TaNK细胞的富集与对肿瘤的免疫反应受损以及对当前ICB治疗的低敏感性有关。该研究发现揭示了TaNK细胞在肿瘤中的潜在作用,并为NK细胞免疫疗法的合理设计提供了参考。图5 TaNK细胞与临床结局的关系6. TME塑造肿瘤浸润NK细胞功能的潜在介质为了深入了解NK细胞在TME中的调控程序,我们利用CellPhoneDB来探测NK细胞和其他CD45+免疫细胞(包括T细胞和髓细胞)之间潜在的细胞-细胞相互作用。与T细胞相比,除肥大细胞外,大多数髓细胞类型与CD56dimCD16hi NK亚群表现出强烈的潜在相互作用(图6A)。特别有趣的是,TaNK细胞被预测通过ANXA1调节多髓细胞类型,ANXA1是一种在炎症反应中与免疫抑制和诱导巨噬细胞重编程相关的蛋白(图6B),这表明功能失调的NK细胞可能具有抑制TME中促炎巨噬细胞的潜力。为了进一步阐明NK细胞来源的ANXA1在巨噬细胞中的作用,作者对肺癌和肝癌的肿瘤样本进行了多重免疫荧光染色,发现了一个ANXA1+ NK细胞群。与远离ANXA1+ NK细胞的巨噬细胞相比,靠近ANXA1+ NK细胞的巨噬细胞表现出较低的活化标志物CD86(图6C、6D)和较高的抗炎标志物转化生长因子β (TGF-β)的表达水平(图6E、6F)。值得注意的是,在树突状细胞(DC)亚群中,LAMP3+ DC,最近表征的成熟cDCs(也称为mregDC)显示出与CD56dimCD16hi NK细胞最强的相互作用潜力(图6A)。多重免疫荧光分析显示,LAMP3+ DCs与NK细胞共定位(图6J)。此外,预测它们之间的相互作用通过IL-15-IL-15受体和NECTIN2-TIGIT相互作用轴介导(图6B)。 重要的是,LAMP3+ DCs在转录组水平上表达免疫群体中最高水平的IL15、PVRL2 (NECTIN2)和PVR(图6G和S6F)。流式细胞术也证实了IL-15在LAMP3+ dc中的高表达(图6H)。IL-15已被确定为维持NK细胞寿命的稳态相关细胞因子,并用于NK细胞输注和体外繁殖。相反,TIGIT作为一种抑制性受体有助于抑制NK细胞介导的免疫反应。此外,在TCGA数据集中,LAMP3+ DCs的丰度与CD56dimCD16hi NK细胞相关(图6I)。 接下来,作者研究了LAMP3+ DCs在肿瘤中的具体调控过程,发现肿瘤浸润的LAMP3+ DCs与邻近非肿瘤组织相比,IL15的表达较低,表明LAMP3+ DCs可能对TME中CD56dimCD16hi NK细胞的激活作用受损。事实上,物理上接近LAMP3+ DCs的NK细胞表达颗粒酶B的水平较低(图6J、6K)。 总之,以上数据分析表明,在TME中,LAMP3+ DCs对CD56dimCD16hi NK细胞的异常调节。图6 在不同肿瘤类型中,LAMP3+ DCs与CD56dimCD16hi NK细胞的关系 7.外周血NK细胞亚群的不同转录组模式通过分析来自35名健康供者的血液来源NK细胞的9个scRNA数据集,能够探测肿瘤患者外周血中NK细胞的特异性改变。作者首先比较了来自健康供体和肿瘤患者的循环NK细胞的转录组特征。循环NK细胞在来自不同数据集的健康供体中表现出高度的相似性(图S7B)。相比之下,对于来自肿瘤患者的循环CD56brightCD16lo细胞,观察到与健康供者的转录组存在显著差异;在所分析的所有癌症类型中,循环CD56dimCD16hi细胞的差异甚至更为显著(图S7A)。值得注意的是,肿瘤患者在NK细胞亚群中表现出显著的组成变化,这种模式似乎是癌症类型特异性的(图S7C)。例如,在结直肠癌、头颈部鳞状细胞癌、肾癌和HCC中,循环CD56brightCD16lo c3-CCL3 NK细胞的比例增加,但在其他被分析的癌症类型中没有增加。接下来,作者将重点放在CD56dimCD16hi c8-KLRC2适应性NK细胞上,该细胞在某些癌症类型如结直肠癌和胃癌中富集(图S7D)。适应性NK细胞被认为是CAR NK细胞的一个有吸引力的来源,因为它们具有增强细胞因子反应的效应特性和对免疫抑制效应的内在抗性特别有趣的是,与其他循环NK细胞相比,这些细胞特异性表达MHC II类基因(图S7E)。作者还检查了这些NK细胞在肿瘤患者中的功能变化,发现与健康供体相比,肿瘤来源的适应性NK细胞的功能基因和MHC II类基因的表达明显更高(图S7F和S7H),这意味着它们在肿瘤患者中处于高度激活状态。作者通过流式细胞术进一步证实,与健康供者相比,HCC患者循环NK细胞中MHC II类分子的高表达(图S7G)。因此,在患者源性适应性NK细胞中上调的基因参与了免疫效应过程的正调控等途径(图S7I)。综上所述,研究分析表明,循环NK细胞参与了肿瘤进展过程中外周免疫环境的系统性变化。图S7患者循环NK细胞的改变(与图1相关) 讨论本研究中,作者收集了大量的单细胞转录组数据,首次在泛癌水平系统地鉴定到了5类CD56brightCD16lo和9类CD56dimCD16hi NK细胞亚群,并详细地刻画了各类群的表型和功能多样性。这些NK细胞可能参与了广泛的抗肿瘤反应,如直接杀死癌细胞、分泌促炎细胞因子和募集其他免疫成分(图7A和7B)。促进NK细胞在实体恶性肿瘤中的浸润一直是开发治疗性NK细胞产品的关键焦点。我们在转录组水平上发现RGS1是组织浸润NK细胞的关键标志物(图7C)。促进NK细胞在实体恶性肿瘤中的浸润一直是开发治疗性NK细胞产品的关键焦点。作者在在转录组水平上发现RGS1是组织浸润NK细胞的关键标志物(图7C)。此外,作者发现了一个潜在功能失调状态的肿瘤富集NK细胞亚群,称为TaNK细胞(图7D)。TaNK细胞的高丰度与多种癌症类型的不良预后和免疫治疗耐药性有关,暗示了其在临床环境中的作用。作者推测,TaNK细胞富集可能反映或影响TME中的肿瘤免疫反应,尽管这些细胞可能不是ICB治疗的直接靶点。本研究的分析和实验证据支持骨髓细胞是NK细胞的核心介质。具体来说,LAMP3+ DCs可以作为关键的调节剂,并可能抑制CD56dimCD16hi NK细胞在TME中的功能,但需要进一步的功能验证 (图7E)。总之,作者通过寻找细胞内在和TME相关的因子,为维持NK细胞在体内的抗肿瘤活性提供了线索。图7研究的主要发现总结本研究聚焦TME中与T细胞功能相似、但具有更直接的识别和杀伤癌细胞能力的NK细胞。作者创新性地整合利用大规模单细胞数据,克服数据整合的多项难点,包括高精度分离NK细胞、去除复杂的批次效应等,首次在泛癌水平系统地鉴定到5类CD56brightCD16lo和9类CD56dimCD16hiNK细胞亚群,并详细地刻画了各类群的表型和功能多样性,揭示了NK细胞中的基因表达模式转变,捕捉了肿瘤免疫微环境的NK细胞亚群组分变化,为未来通过更全面的整合分析手段探索新的生物标志物和治疗靶点提供了助力,也为药物研发提供更准确、更全面的数据支持。参考文献:Tang F, Li J, Qi L, Liu D, Bo Y, Qin S, Miao Y, Yu K, Hou W, Li J, Peng J, Tian Z, Zhu L, Peng H, Wang D, Zhang Z. A pan-cancer single-cell panorama of human natural killer cells. Cell. 2023 Aug 15:S0092-8674(23)00849-8. doi: 10.1016/j.cell.2023.07.034. Epub ahead of print. PMID: 37607536.识别微信二维码,添加生物制品圈小编,符合条件者即可加入生物制品微信群!请注明:姓名+研究方向!版权声明本公众号所有转载文章系出于传递更多信息之目的,且明确注明来源和作者,不希望被转载的媒体或个人可与我们联系(cbplib@163.com),我们将立即进行删除处理。所有文章仅代表作者观点,不代表本站立场。
细胞疗法临床研究
2023-09-20
·生物谷
来自北京大学等机构的科学家们通过研究解析了NK细胞在多种不同癌症类型和组织中的异质性,并发现了一类具有异常抗肿瘤功能的NK细胞亚型,其能在肿瘤微环境中特异性地生长。
自然杀伤性细胞(NK cells)在机体抵御肿瘤进展的先天性免疫反应中扮演着不可或缺的角色,近日,一篇发表在国际杂志Cell上题为“A pan-cancer single-cell panorama of human natural killer cells”的研究报告中,来自北京大学等机构的科学家们通过研究解析了NK细胞在多种不同癌症类型和组织中的异质性,并发现了一类具有异常抗肿瘤功能的NK细胞亚型,其能在肿瘤微环境中特异性地生长。
NK细胞因能直接杀死癌细胞而得名,如今其已经成为了免疫疗法的有力竞争者,并在血液癌症疗法中展现出了非凡的疗效,然而,NK细胞在不同组织微环境中的表型和功能各不相同,其异质性也给其在实体瘤疗法中的应用带来了一定的挑战。多年以来,研究者Tian教授等人一直在研究不同组织中NK细胞的异质性,这项研究中,他们收集了一个广泛的单细胞转录组数据集,其中涵盖24种癌症类型,包括来自716名患者和47名健康个体共1223份肿瘤样本。
研究人员首次在全面泛癌水平上识别出了CD56brightCD16lo NK细胞的5个不同亚型和CD56dimCD16hi NK细胞的9个亚型,同时对这些亚型的表型和功能多样性进行了细致地分析。通过整合这一广泛的数据集,研究人员观察到了不同的癌症类型中NK细胞亚型组成的偏好情况,值得注意的是,NK细胞亚型在肿瘤中、临近组织和外周血液中的分布表现出了明显的差异。利用先进的生物信息学技术,研究人员发现,RGS1基因在非血液NK细胞中会高度表达,在转录水平上,相比传统的组织残留标志物而言,RGS1表现出了显著的特异性和灵敏度。
科学家识别出异常的自然杀伤性细胞亚型。
图片来源:Cell (2023). DOI:10.1016/j.cell.2023.07.034
通过探究肿瘤微环境,研究人员发现,DNAJB1+CD56dimCD16hi NK细胞在肿瘤中往往会高度富集,对这一群体进行分析表明,其表型功能失调,细胞毒性会发生降低,同时抑制性受体增加了,压力相关的蛋白水平也提高了。这种名为肿瘤相关NK细胞(TaNK cells,Tumor-associated NK cells)的亚型打破了科学家们对NK细胞丰度越高对肿瘤患者也有益的传统认知,相反,TaNK细胞与多种癌症类型患者的不良预后及对免疫疗法存在明显的抵抗力之间存在着密切关联。
此外,研究人员还发现,LAMP3+树突状细胞(DCs)是NK细胞功能的关键调节因子,空间分布数据分析结果表明,靠近LAMP3+ DCs的NK细胞的细胞毒性活性会下降,这一观察性的研究结果表明,LAMP3+ DCs或许会对肿瘤微环境中的NK细胞功能产生异常的调节性效应。综上,本文研究结果或为科学家们理解基于NK细胞的癌症免疫力提供了新的见解,并强调了NK细胞亚群作为治疗性靶点的潜在临床用途。(生物谷Bioon.com)
原始出处:
Fei Tang,Jinhu Li,Lu Qi, et al. A pan-cancer single-cell panorama of human natural killer cells, Cell (2023). DOI: 10.1016/j.cell.2023.07.034
免疫疗法细胞疗法临床研究
2023-09-11
·生物谷
综上所述,这一工作创新性地整合利用大规模单细胞数据,揭示了NK细胞中的基因表达模式转变,捕捉了肿瘤免疫微环境的NK细胞亚群组分变化,为未来通过更全面的整合分析手段探索新的生物标志物和治疗靶点提供了助力
自然杀伤(Natural Killer, NK)细胞是免疫系统重要成员,被誉为肿瘤的天然杀手,以其具备直接杀伤肿瘤细胞的功能而命名。近年来,随着肿瘤免疫治疗的兴起,基于NK细胞的免疫疗法已成为新兴主力军,在血液瘤治疗领域大放异彩,但对实体瘤的治疗却进展缓慢。由于NK细胞是异质性的群体,在不同组织微环境中由表型和功能各异的亚群组成不尽相同。因此,对肿瘤组织NK细胞开展全面系统的研究有利于为NK细胞免疫疗法扫清障碍。
中国科学技术大学田志刚和彭慧课题组多年来聚焦NK细胞的组织异质性,在肝脏、肠道、皮肤等组织发现特殊NK细胞群体,近来与北京大学张泽民课题组等合作,系统描绘NK细胞在不同癌症类型和组织之间的异质性,发现了肿瘤微环境特异富集、杀伤功能异常的NK细胞亚类,揭示了NK细胞与微环境中其他组分的潜在调控关系。该研究成果以“A Pan-Cancer Single Cell Panorama of Human Natural Killer Cells”为题在线发表在《Cell》上。在该研究中,彭慧教授为共同通讯作者。
研究者收集整理了大量公开的单细胞转录组数据,涉及24种癌症类型,包括了来自716名患者和47名健康对照的1223个样本的NK细胞单细胞表达谱数据,首次在泛癌水平系统地鉴定到了5类CD56brightCD16lo和9类CD56dimCD16hiNK细胞亚群,并详细地刻画了各类群的表型和功能多样性。
基于整合的数据资源,研究者发现NK细胞的亚群组成在不同癌症类型间表现出了明显的偏好性,肿瘤、癌旁组织和外周血中的NK细胞亚类分布也具有明显的差异。通过生物信息技术筛选,发现RGS1特异高表达在非血液来源的NK细胞上。相比于经典的组织驻留特征基因,RGS1在转录组水平表现出更高的特异性和灵敏度,并且在泛癌种水平上都具有优秀的性能。进一步关注肿瘤微环境,研究者发现肿瘤组织中高度富集一群DNAJB1+ CD56dimCD16hi NK细胞。数据分析发现该群细胞具有功能失调的表型,包括杀伤性下降、抑制受体上升、高表达应激反应相关蛋白等,因此将其命名为肿瘤相关NK细胞(Tumor-associated NK cells, TaNK cells)。与经典认知的NK细胞的更高丰度有益于肿瘤患者的生存状态不同, TaNK细胞的富集与多种癌症类型的不良预后、及免疫治疗的耐药显著相关。这些发现表明了TaNK细胞具有重要的生物学和临床应用价值,为后续开发NK细胞相关的免疫治疗方法提供了新的思路。此外,研究者发现髓系细胞尤其是LAMP3+ DCs是NK细胞的重要调控因子。进一步的生物信息分析结合空间分布定量发现,靠近LAMP3+ DCs的NK细胞表现出更低的杀伤活性,这说明LAMP3+ DCs在肿瘤免疫微环境中对NK细胞功能有着潜在的异常调节作用。
研究主要发现示意图
综上所述,这一工作创新性地整合利用大规模单细胞数据,揭示了NK细胞中的基因表达模式转变,捕捉了肿瘤免疫微环境的NK细胞亚群组分变化,为未来通过更全面的整合分析手段探索新的生物标志物和治疗靶点提供了助力,也为药物研发提供更准确、更全面的数据支持。
免疫疗法细胞疗法临床研究
100 项与 HER2.taNK(NantKwest) 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 复发性胶质母细胞瘤 | 临床1期 | 德国 | 2017-12-01 | |
| 膀胱癌 | 临床前 | 美国 | 2015-07-16 | |
| 乳腺癌 | 临床前 | 美国 | 2015-07-16 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
