预约演示
更新于:2025-07-14
Pembrolizumab biosimilar(Sandoz)
帕博利珠单抗生物类似药(Sandoz)
更新于:2025-07-14
概要
基本信息
在研机构 |
非在研机构- |
权益机构- |
最高研发阶段临床1期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
结构/序列
Sequence Code 93660H

当前序列信息引自: *****
Sequence Code 93670L

当前序列信息引自: *****
关联
8
项与 帕博利珠单抗生物类似药(Sandoz) 相关的临床试验CTIS2024-513160-25-00
An open-label, multi-center, single arm, rollover study of GME751 (proposed pembrolizumab biosimilar) in participants who are eligible for continued pembrolizumab treatment after participation in CGME751A12101 or CGME751A12301 studies. - CGME751A12302
开始日期2024-11-15 |
申办/合作机构- |
CTIS2023-506881-30-00
- CGME751A12101
开始日期2024-05-15 |
申办/合作机构- |
NCT06153238
A Randomized, Double-blind, Parallel-group Study to Compare Pharmacokinetics of GME751 (Proposed Pembrolizumab Biosimilar) and US-licensed and EU Authorized Keytruda® in Participants With Stage II and III Melanoma Requiring Adjuvant Treatment With Pembrolizumab
The purpose of this study is to investigate the pharmacokinetic (PK) similarity and efficacy, safety, and immunogenicity of GME751 compared with Keytruda® (pembrolizumab) in subjects with resected advanced melanoma requiring adjuvant treatment with pembrolizumab.
开始日期2024-05-15 |
申办/合作机构 |
100 项与 帕博利珠单抗生物类似药(Sandoz) 相关的临床结果
登录后查看更多信息
100 项与 帕博利珠单抗生物类似药(Sandoz) 相关的转化医学
登录后查看更多信息
100 项与 帕博利珠单抗生物类似药(Sandoz) 相关的专利(医药)
登录后查看更多信息
4
项与 帕博利珠单抗生物类似药(Sandoz) 相关的新闻(医药)2025-05-26
·动脉网
随着精准医疗与技术创新的不断演进,肿瘤药物研发正在经历深度重构。从靶点聚焦、疾病领域分布,到机构参与模式与技术路径,全球研究版图愈加清晰。特别是在2025年AACR年会公布的研究汇总分析后,核心趋势已逐渐呈现:传统靶点依旧强势,新兴靶标加速突破,机构分工更趋专业化,AI技术全面渗透药物发现与诊疗预测环节。本篇行业研究基于AACR 2025发布的全量摘要数据,系统梳理了全球肿瘤研发三大维度:(1)疾病与靶点研究热点,(2)机构类型与技术趋势,以及(3)AI在肿瘤研究中的融合应用。通过对7100余篇AACR 2025参会文章的内容归类与解析,本文旨在帮助读者快速掌握全球肿瘤研发的核心焦点、地区与机构间的差异化布局、前沿靶点的落地路径与AI技术赋能的实际场景。01全球肿瘤研究热点方向1.1 实体瘤为研究主战场,血液系统次之根据AACR 2025年会公布的研究统计数据,实体瘤研究占比高达89.14%,远超血液系统肿瘤(8.34%)及其他疾病类别(如代谢性疾病0.89%、免疫性疾病0.66%)[1]。这一研究格局与全球肿瘤负担相吻合。GLOBOCAN 2020报告显示,肺癌、乳腺癌和结直肠癌等固态瘤种占新发癌症病例的60%以上[2],实体瘤仍是当前抗肿瘤药物开发和基础研究的核心领域。来源:根据AACR 2025大会网站信息,医药魔方的数据整理1.2 系统性肿瘤研究重心分布与临床负担吻合对实体瘤进一步分类发现,乳腺及妇科肿瘤(26.7%)与消化系统肿瘤(26.7%)并列为研究比例最高的领域,其次为呼吸系统肿瘤(16.1%)和泌尿生殖系统肿瘤(12.1%)[1]。这与全球高发癌种排名基本一致,其中乳腺癌为女性最常见肿瘤(占11.7%),结直肠癌和肺癌紧随其后[2]。在血液系统肿瘤中,白血病研究占比达52.8%,遥遥领先于淋巴瘤(27.4%)与浆细胞瘤(15.4%)。近年来,CAR-T、BiTE以及Bcl-2抑制剂等靶向策略的突破推动了白血病治疗手段的快速演进,呈现出细胞疗法与靶向药物双线并进的格局。感染相关疾病领域,HPV病毒致癌机制相关研究占据主导地位(46.2%),远超EBV相关研究(5.1%)。近年来研究焦点已由预防型HPV疫苗转向治疗性疫苗与免疫联合疗法开发,其在宫颈癌、头颈癌等肿瘤治疗中展现潜力。来源:根据AACR 2025大会网站信息,医药魔方的数据整理在代谢相关疾病中,研究集中于Ⅰ/Ⅱ型糖尿病(36.4%)与肥胖(30.3%)背景下的肿瘤易感性,其余如高胆固醇血症(15.2%)、胰岛素抵抗(6.1%)研究占比相对较小。流行病学研究证实,肥胖和Ⅱ型糖尿病显著增加胰腺癌和结直肠癌的发病风险[3],提示代谢失衡是重要的肿瘤致病背景,亦为早筛与干预提供新方向。免疫相关疾病研究中,自身免疫病(41.3%)与炎症性肠病(IBD,23.9%)最受关注。IBD患者的结直肠癌风险是常人的2倍以上[4],慢性炎症对肿瘤微环境的重塑作用正成为研究热点。该类研究有助于进一步解析免疫调控与肿瘤发生之间的关联。在遗传性癌症综合征研究中,林奇综合征(64.3%)为最主要研究对象,其在结直肠癌和子宫内膜癌中的早筛与风险管理已纳入临床指南。遗传性肿瘤研究强调对高危人群的早期干预与个体化防控策略。肿瘤相关并发症研究则聚焦于治疗相关毒性(71.4%),如细胞毒性、免疫不良反应等,远高于放射诱导性纤维化(28.6%)。随着治疗技术的升级,并发症管理日益成为优化临床方案不可忽视的部分。来源:根据AACR 2025大会网站信息,医药魔方的数据整理1.3 癌种亚型精细化研究趋势明显在乳腺及妇科肿瘤中,乳腺癌研究占比达59.3%,为最主要研究方向,卵巢癌(18.3%)与三阴性乳腺癌(TNBC,8.8%)紧随其后。三阴性乳腺癌因缺乏激素及HER2靶点,正积极探索T细胞引导疗法与抗体偶联药物(ADC)策略[5]。乳腺癌亚型研究逐步从分子分型向精准靶向与免疫联合推进。在消化系统肿瘤中,结直肠癌(38.4%)和胰腺癌(32.1%)为研究重点。胰腺癌因五年生存率低于10%,被广泛视为“难治性实体瘤”的攻坚方向;肝癌(13.3%)与胃癌(9%)研究关注亦不断升温。呼吸系统肿瘤以非小细胞肺癌(NSCLC,45.5%)、肺腺癌(13.1%)及小细胞肺癌(SCLC,12.2%)为主要研究对象,EGFR突变、ALK融合等经典靶点持续受到关注。来源:根据AACR 2025大会网站信息,医药魔方的数据整理泌尿生殖系统肿瘤中,前列腺癌(62.4%)研究占比最高,肾癌(15.1%)、膀胱癌(13.9%)次之。PSMA靶向放射性治疗与AR螺旋酶抑制剂相关成果获得FDA优先审评资格[6],AR与PSMA双靶点策略成为前列腺癌治疗焦点。此外,肉瘤、神经系统与头颈部肿瘤的研究各有聚焦,主要关注稀有靶点、特定分子变异及免疫相关机制。血液系统肿瘤依然以白血病(49.6%)与淋巴瘤(25.3%)为主,罕见亚型研究正在获得更多关注,推动治疗手段与生物标志物的进一步多样化与精准化。来源:根据AACR 2025大会网站信息,医药魔方的数据整理1.4 TOP10 靶点研究:“广谱核心+系统特异”并进消化系统肿瘤靶点覆盖传统驱动基因(KRAS、EGFR、TP53)、免疫通路(PD-1/PD-L1)及关键信号通路(PI3K/AKT、β-catenin),展现“靶向+免疫+信号通路联合”的发展趋势。KRAS为胰腺导管癌和结直肠癌的核心驱动基因,G12C抑制剂sotorasib联合panitumumab方案已获FDA批准用于KRAS G12C突变的转移性结直肠癌[7]。TP53为最常突变的抑癌基因,突变率在消化系统肿瘤中显著偏高[8]。PD-1/PD-L1抑制剂在MSI-H/dMMR型结直肠癌中作为一线治疗已被证实有效,KEYNOTE-177试验中pembrolizumab改善了生存期与耐受性[9]。肺癌聚焦EGFR和KRAS等传统靶点,同时引入免疫策略及新靶点DLL3。第三代EGFR TKI(如lazertinib)联合抗体amivantamab已获批,开启“TKI+抗体”治疗时代[10]。KRAS G12C抑制剂在NSCLC中率先获批,并拓展至其他亚型。DLL3为小细胞肺癌特异性靶点,双特异抗体和CAR-T疗法正在临床早期阶段展现潜力。来源:根据AACR 2025大会网站信息,医药魔方的数据整理泌尿生殖系统肿瘤以AR和PSMA为前列腺癌核心靶点,同时扩展至PD-L1和Nectin-4。Pluvicto®(PSMA靶向放射配体)已被批准用于mCRPC[11]。膀胱癌中Nectin-4靶向ADC(如enfortumab vedotin)临床获益显著。乳腺与妇科肿瘤采用“信号通路+DNA修复+免疫”组合策略,HER2(23.9%)与ER(13.2%)为两大核心靶点。来源:根据AACR 2025大会网站信息,医药魔方的数据整理罕见实体瘤聚焦免疫检查点与融合蛋白,尤文氏肉瘤的EWS-FLI1已成为关键候选靶点。PD-1(24.7%)、p53(12%)、CTLA-4(10.1%)等位居研究前列。BRAF在黑色素瘤中应用广泛,PD-1与CTLA-4联合治疗已成为标准方案。神经系统肿瘤主要靶向EGFR、p53、MGMT及IDH1,构建精准治疗组合。头颈部肿瘤则结合免疫靶点与病毒抗原,探索表观遗传路径。血液系统则聚焦Bcl-2调控、CD19/BCMA CAR-T与双抗,以及BTK/FLT3信号通路。来源:根据AACR 2025大会网站信息,医药魔方的数据整理综上,p53与PD-1/PD-L1在六大系统中均位列TOP10,显示广泛的治疗适用性。KRAS与EGFR在消化与呼吸系统中均为核心靶点,反映其在实体瘤中的共性机制。同时,各系统也呈现独特靶点:AR/PSMA限于前列腺癌,EWS-FLI1专属尤文氏肉瘤,MGMT/IDH1聚焦神经系统,CD19/BCMA聚焦血液肿瘤,HPV抗原集中于头颈部肿瘤,明确揭示了肿瘤靶向治疗的“共性+特异”结构。1.5 见解:TOP3 系统肿瘤靶点分布与技术趋势消化系统肿瘤(胃肠、肝胆胰等):代表性靶点包括EGFR、HER2、KRAS、BRAF、PI3K、VEGF等传统靶点;新兴靶点如Claudin18.2(柯德素2)和GPC3在胃癌、肝癌中受关注[1]。例如,Claudin18.2抗体zolbetuximab已获批用于胃食管腺癌[12],对应研究也在AACR等会议频繁报道。KRAS突变(尤其G12C/G12D)在胰腺癌和结直肠癌中高发,目前针对KRAS G12C的抑制剂已上市,而新型G12D抑制剂(如zoldonrasib、MRTX1133)正显示出临床前和I期疗效[13,14]。免疫检查点(如PD-1/PD-L1)和新型ADC靶点(如HER2、TROP2)也占据主要研究阵地。总体来看,消化道肿瘤靶向研发既延续了对EGFR、HER2、KRAS等传统靶点的关注,同时积极拓展Claudin18.2、GPC3、FGFR2(胆管癌)等新靶点的潜力。呼吸系统肿瘤(主要指肺癌):关键靶点包括EGFR、ALK、ROS1、BRAF、MET、RET、KRAS以及免疫靶点PD-1/PD-L1等传统目标;同时出现诸如HER2突变、NRG1融合(HER3相关)、TIGIT、DLL3等新靶点。典型案例是肺癌:第3代EGFR抑制剂(如lazertinib)已与双特异抗体amivantamab联合获FDA批准[15];KRAS G12D抑制剂zoldonrasib呈现初步临床活性[16];HER3靶向双特异抗体zenocutuzumab(针对NRG1融合肿瘤)实现了临床突破[17]。新药开发方面,研究重点正从传统EGFR/ALK等靶点拓展到KRAS变体、HER2/HER3信号通路以及各种免疫通路的联合抑制。可见,肺癌领域的靶点分布呈现“老靶点加新靶点并行”的趋势。泌尿生殖系统肿瘤(前列腺、膀胱、肾脏等):前列腺癌以雄激素受体(AR)为传统靶点,近期PSMA(前列腺特异膜抗原)放射性核素治疗获得FDA批准(Pluvicto™)[18],展示了新靶点带来的治疗潜力。膀胱癌研究中,FGFR3突变/融合常见,对应靶向药如pemigatinib获批;Ras/MAPK和PD-1免疫靶点也在临床试验中占据重要地位。肾癌则以VEGF/VEGFR(抗血管生成)和mTOR路径为经典靶点,近年来对免疫检查点和新型靶点如HIF-2α抑制剂(belzutifan)等展开布局。总的来看,泌尿生殖系统肿瘤中,传统靶点仍主导(如AR、VEGFR、FGFR),但新靶点(PSMA、HIF-2α、各种免疫检查点)研究热度显著上升。1.6 见解:传统 vs 新兴靶点:研发热度与创新潜力传统靶点(KRAS、EGFR、p53等):这些靶点历经多年研究,目前在研发中仍占据主导地位。KRAS家族是典型代表,近年来G12C抑制剂(sotorasib、adagrasib)成功上市,研究重心逐渐向G12D等其他变体转移。例如,zoldonrasib作为首个口服KRAS G12D抑制剂,在KRAS G12D突变的NSCLC患者中取得了客观缓解[16];MRTX1133在胰腺癌模型中诱导深度肿瘤退缩[14]。EGFR靶向仍是肺癌研究热点,新一代EGFR抑制剂联用双特异抗体方案已进入临床并获批[15]。p53作为经典肿瘤抑癌基因,其直接靶向仍具挑战性,当前更多研究集中在p53基因补偿疗法或相关通路(如MDM2)的干预。在药企布局方面,大型制药公司持续推进这些经典靶点的升级药物和组合疗法,例如多靶点抑制剂和新型抗体偶联药物等。新兴靶点(BCMA、PSMA、CD19等):这些靶点在近年研究中活跃度显著上升,创新潜力巨大。以血液肿瘤为例,BCMA靶向治疗(CAR-T和双特异抗体)已成为多发性骨髓瘤治疗的焦点。Roche/Poseida开发的全新异体CAR-T产品P‑BCMA‑ALLO1在复发/难治MM患者中展示了91%总缓解率[19],显示出比单靶向更强的疗效。PSMA靶向策略在前列腺癌表现亮眼,^177Lu-PSMA放射性核素治疗(Pluvicto)已获准用于晚期转移性前列腺癌并正在拓展至一线应用[18]。B淋巴瘤靶点CD19则通过CAR-T和双靶CAR-T等新技术持续创新,合作开发的双抗CD19/CD20 CAR-T首次实现异体双靶CAR-T临床应用[19]。这些新靶点的研究通常伴随着新型疗法平台(如CAR-T、双抗体、ADC、多特异抑制剂)同步推进,使其在AACR等会议上成为讨论热点。此外,ADC、肿瘤疫苗、CRISPR免疫治疗等技术趋势正在为传统和新靶点研究注入新动力,激发更多创新。1.7 见解:TOP10研究机构画像AACR 2025中排名前列的机构主要包括MD安德森癌症中心、Memorial Sloan Kettering(MSKCC)、Dana-Farber癌症研究所、约翰霍普金斯大学、NCI/NIH等。这些中心往往拥有多学科整合的平台和丰富的临床资源。例如,MSKCC领导的免疫疗法研究组在错配修复缺陷(MMRd)实体瘤中报道了PD-1抗体dostarlimab的新辅助疗法,80%患者完成治疗后无需手术[20];其团队还获得AACR奖项以表彰MSK-IMPACT基因测序平台的贡献[21]。MD安德森则在PARP抑制剂联合免疫疗法、数字孪生模型等领域持续发力,本次报告了针对BRCA突变患者的PARP+PD-1组合试验早期数据[20]。Dana-Farber在头颈癌、肺癌和乳腺癌等多种疾病中保持领先地位,重点研究方向包括HPV阴性头颈癌的新辅助免疫治疗、KRAS突变肺癌联合通路抑制[22]以及乳腺癌抗耐药联合方案等。以上机构的共同优势在于强大的科研实力、多中心临床试验网络以及跨学科合作(如免疫学、基因组学与临床研究结合),使其在AACR发文中表现突出。来源:根据AACR 2025大会网站信息,医药魔方的数据整理02研发机构类型、地区与创新分布2.1 机构类型与地区:大学领跑,北美为重心在AACR 2025大会披露的研究数据中,高校机构占据主导地位,占比达35.6%,凸显其在基础研究与创新路径探索中的重要作用。企业和医院分别占据22.9%和22.5%,体现出产业转化和临床验证作为肿瘤研发核心驱动的双轮结构。独立研究机构占比16.3%,展现其在方法学、平台开发与基础生物学中的支撑性角色。从地区分布来看,北美以57.5%的占比稳居全球肿瘤研究高地,其中美国的研究机构公布研究数量超过4700多份,远超全球其他国家,稳居全球第一;亚洲(21.9%)与欧洲(17.5%)紧随其后,中国以758的公布数量占据全球第二,其后是韩国388;英国、法国、德国、西班牙和意大利均位列全球前十,是欧洲癌症研究关注度和投入较高的区域,与当地的人口体量具有较强的相关性。相较之下,大洋洲(1.3%)、南美(1.0%)及非洲(0.9%)占比较低,提示肿瘤研究在全球尚有地域拓展与合作潜力。来源:根据AACR 2025大会网站信息,医药魔方的数据整理2.2 药物品种研发趋势:抗体与小分子双核驱动,新体系加速拓展大学、企业、医院与独立研究机构在肿瘤药物研发路径上呈现“双核(小分子+大分子)主导、平台技术融合”的共性框架,并在具体布局上各具特点。高校倾向于以小分子为主(占比约40%),其中约30%集中在TKI与传统靶向药,另有25%左右聚焦于ADC、双抗、PROTAC与AI辅助筛选等前沿平台,体现其基础研究与技术验证双向优势。企业则呈现出“30%小分子+20%抗体+20%ADC+10%免疫检查点”四足并进结构,同时在靶向蛋白降解与放射性配体等新兴机制(10%)方面快速推进商业化。医院机构更聚焦临床应用,抗体药物(25%)与免疫调节剂(15%)布局靠前,搭配小分子(30%)与细胞/基因疗法(10%),体现其对一线治疗与CAR-T平台的双重重视。研究机构中,“其他”创新平台(占比约35%)居首,其次为小分子(30%)和ADC(15%),更偏重高通量靶点筛选与机制创新。整体来看,四类机构在稳固传统路径的同时,正加速布局多模态、跨平台的新兴疗法,显示肿瘤药物研发正向“靶点+平台”双驱动战略演进。来源:根据AACR 2025大会网站信息,医药魔方的数据整理2.3 各类机构聚焦的TOP10靶点分析在靶点选择上,PD1、PDL1、EGFR、HER2、KRAS与p53为各类机构共同关注的核心靶点,反映免疫检查点与驱动突变位点在肿瘤治疗中的长期战略地位。大学倾向于围绕MYC、AR、Akt、PI3K等基础生物学靶点进行探索,突出其在信号转导、细胞周期和内分泌肿瘤机制研究中的学术深度。企业则集中于CD3、TROP2、CTLA-4等免疫靶点,布局双抗、CAR-T、ADC等商业潜力明确的新兴治疗形式。医院更关注CDKN2A等与患者分型和临床转化密切相关的靶点,体现其桥接基础与临床的优势。研究机构则重点发力Ras、Akt等信号通路关键节点,强化对肿瘤依赖机制的机制性解析与早期验证。来源:根据AACR 2025大会网站信息,医药魔方的数据整理2.4 全球TOP20 MNC的研发趋势全球TOP 20跨国药企(MNC)在药物类型方面,小分子药物仍为主导(33%),但大分子(19.7%)及技术驱动类药物(7.1%)比例持续上升,体现出研发模式由传统单一平台向多技术耦合的方向演进。在疾病布局上,消化系统肿瘤、乳腺及妇科肿瘤、呼吸系统肿瘤为三大重点领域,分别占18%、18%和16.6%,对应全球肿瘤负担高发趋势。在靶点选择上,PD1、EGFR、PDL1、HER2的高频布局(分别为22、19、18和14次)验证其在免疫+靶向双线战术中的核心地位。表:全球TOP20 MNC公司名单来源:根据美国《制药经理人》公布信息整理来源:根据AACR 2025大会网站信息,医药魔方的数据整理在代表性药物方面,罗氏的选择性ER降解剂 giredestrant(用于HR+/HER2–乳腺癌)进入III期;阿斯利康的HER2-ADC Enhertu、Imfinzi在乳腺和肺癌中不断拓展适应症;诺华的放射性配体疗法Pluvicto(靶向PSMA)在前列腺癌中改善疗效并已上市[23]。此外,强生的EGFR/MET双抗Rybrevant联合方案和辉瑞的BRAF抑制剂Braftovi也体现出靶点协同策略的临床价值。在新兴技术平台方面,Arvinas的BCL6靶向PROTAC(ARV-393)进入I期临床,BMS的AR降解剂CC-199正在临床前推进[24-27];CRISPR基因编辑疗法Casgevy(由Vertex与CRISPR Therapeutics联合开发)2023年在英国获批上市,成为首个CRISPR肿瘤疗法产品,代表靶点治疗正迈入基因级干预新阶段[28]。此外,多家药企在CAR-T、ADC以及靶向蛋白降解平台上的联合探索,正重塑下一代肿瘤治疗范式。2.5 中国TOP20药企的研发趋势中国头部药企近年来快速崛起,展现出“生物制品主导+新兴技术突破”的研发格局。统计显示,其在药物类型上对大分子药物(抗体、融合蛋白等)投入占比高达65%,形成对单抗、双抗、ADC等产品形态的研发优势;靶向蛋白降解类药物比例达15%,显示出在新机制药物方面的积极布局,尤其是在PROTAC和分子胶方向开始具备一定储备。在适应症选择上,消化系统肿瘤、乳腺及妇科肿瘤、呼吸系统肿瘤并列为重点关注方向,均占比24.1%,贴合国内肿瘤发病谱的高发趋势。在靶点层面,EGFR和PD-L1分别被提及4次和2次,反映出对经典靶点的持续关注。表:TOP20 中国制药公司名单来源:根据搜狐医药, 美国《制药经理人》,上市公司财报信息整理来源:根据AACR 2025大会网站信息,医药魔方的数据整理近年来,中国药企不断提升原始创新能力。以恒瑞医药为例,其HER2-ADC SHR-A1811已被纳入国家突破性治疗品种名单,并拥有10余个差异化ADC项目处于临床阶段[29]。华东医药则聚焦胃肠道肿瘤抗原,如CDH17/CLDN18.2双靶点ADC VBC108展现出双通路协同激活潜力[30]。在免疫治疗方面,国产首个PD-1/CTLA-4双抗“卡度尼利单抗”已上市,标志国产免疫联合策略进入成熟应用阶段[31]。PD-1单抗方面,君实生物、信达生物等头部企业已构建稳定管线;此外,融合蛋白(如艾加莫德α)和CAR-T/ADC项目也在“国产替代”逻辑下快速推进[32]。根据医药魔方数据统计,2016–2023年间,中国TOP20药企累计获批新药88款,其中64%聚焦肿瘤,41%为自主研发,59%通过引进与合作完成[33]。这表明在满足国内市场需求的同时,中国药企正通过国际合作与平台搭建,向更高水平的原始创新迈进。2.6 TOP20 国际科研机构的研究趋势全球领先科研机构展现出药物研发平台化、多样化的策略特征。在药物类型方面,小分子与大分子药物分别占比30.7%和10.8%,技术驱动类(如蛋白质降解、递送系统)占比7.2%,免疫调节剂与细胞/基因疗法分别为6.6%和6.2%,形成以靶点机制研究为核心、以平台融合为方向的多模态布局。在疾病关注上,消化系统肿瘤(22.2%)、乳腺及妇科肿瘤(19.7%)与呼吸系统肿瘤(12.1%)位列前三,反映出科研机构在贴合流行病学趋势的基础上,优先布局高发高致死性瘤种。在靶点层面,PD1与p53并列为最常被研究靶点(均被提及46次),凸显其在免疫微环境与基因稳定性机制研究中的基础性地位。表:全球TOP20 科研机构来源:根据《新闻周刊》和Statista公司公布的全球权威肿瘤治疗机构的信息整理来源:根据AACR 2025大会网站信息,医药魔方的数据整理2.7 TOP20 中国科研机构的研究趋势中国顶尖科研机构在肿瘤药物研究中同样呈现出平台多元、机制深化的趋势。在药物类型上,小分子仍占主导地位(28.5%),大分子(16.3%)与细胞/基因疗法(6.5%)占比逐年增长,反映出对前沿治疗手段的快速采纳。在疾病布局上,消化系统肿瘤(21.7%)、乳腺及妇科肿瘤(19.7%)和呼吸系统肿瘤(12.8%)依然是主力方向,研究聚焦与国际趋势保持一致。在靶点选择方面,PD-L1与EGFR并列第一(均被提及10次),显示出对免疫检查点和经典驱动基因的持续兴趣与深入研究。表:中国TOP20 科研机构来源:复旦大学医院管理研究所发布的《2023年度中国医院排行榜》和《2024年度中国卫生健康统计年鉴》的信息整理来源:根据AACR 2025大会网站信息,医药魔方的数据整理见解:国际与中国头部科研机构靶点研究趋势分析国际科研机构广泛应用CRISPR筛选、多组学集成与人工智能辅助识别,推动新靶点发现体系化发展。英国Wellcome Sanger研究所联合EMBL-EBI发起的“癌症依赖性图谱”项目,通过对20,000基因进行CRISPR敲除筛选,在300余个癌症模型中建立靶点优先级评分体系[34]。NCI主导的CPTAC项目通过蛋白质组+转录组一体化分析,已发现超2800个潜在可成药靶点[35]。AI也被广泛用于分子网络建模与靶点预测,在精准治疗策略中发挥基础作用[36]。这类成果多以开放数据共享形式发布,并通过Open Targets等联盟促进产业界转化。中国科研机构亦快速引入上述工具体系,部分团队已系统性开展CRISPR筛选、单细胞转录组测序与AI辅助药靶预测工作[37]。政策层面,《国家重大新药创制专项》推动“临床-实验室-企业”三位一体协同攻关,CPTAC数据库等国际资源也逐步向国内开放,助力靶点资源共享与本地化挖掘。2.8 共性与差异:全球与中国研发布局比较共性方面,全球与中国科研机构在靶点聚焦和平台技术上方向趋同,广泛研究PD-1/PD-L1、EGFR、HER2等核心靶点,并采用ADC、双抗、细胞治疗等多种形式落地药物创新;均积极引入蛋白质组学、AI算法与CRISPR筛选等技术手段,推进基础研究向药物开发的高效转化。差异方面,国际头部科研机构与药企依托全球资源,更早布局如KRAS、BCL6、SMARCA2/4等高壁垒靶点,并向PROTAC与CRISPR疗法等未来技术延伸。中国机构起步聚焦“国产替代”路径,以PD-(L)1等成熟靶点切入,近年转向CDH17、GPC3等差异化抗原及AI靶点筛选探索,形成“国产可及+局部突破”的组合策略。此外,跨国企业推动全球同步开发,而中国团队则以本地市场为核心,逐步对接国际标准与临床试验网络[38]。总体而言,中外研发体系在靶点布局和技术采纳上日趋融合,但在策略上各有侧重:国际机构重体系化与广度,中国团队则以速度与灵活机制加速差异化创新,构建“追赶+突围”的双路径体系。03AI赋能肿瘤研究的全球格局3.1 地区与机构画像在AACR 2025大会展示的研究中,人工智能(AI)已成为推动肿瘤学精准化发展的重要技术引擎。总体来看,美国在AI肿瘤研究中保持绝对领先地位,共计发布113篇相关摘要,占全球总量超过一半,延续了其在传统靶点开发与机构产出中的主导态势[39]。韩国(23篇)与英国(15篇)分列第二与第三,体现出亚洲和欧洲国家在AI医疗技术方面的政策支持和研发投入日益加强。中国以11篇排名第四,相较其在传统肿瘤研究中占据的区域份额(亚洲21.9%),在AI领域仍有广阔提升空间。机构方面,美国国立癌症研究院(NCI, 8篇)和芝加哥大学(7篇)为AI肿瘤研究产出最多的机构,其在传统免疫靶点研究中的高活跃度亦有所延续。MD Anderson癌症中心和三星医疗中心则体现出临床型机构在数据驱动医学中的多模态融合能力:前者建立肿瘤学数据科学研究所(IDSO),开发基于深度学习的HER2自动评分系统和肺癌预后AI模型[40],后者则联合KAIST构建AI数字病理平台,并推进多模态影像学研究,覆盖呼吸、消化系统等核心疾病领域[41]。这些机构的共同特点是拥有高度结构化的医疗数据和多学科协作机制,为AI模型的临床转化提供坚实基础。来源:根据AACR 2025大会网站信息,医药魔方的数据整理表:头部机构近3年内AI研发布局案例来源:根据AACR 2025大会网站信息,National Cancer Institute, Pubmed和机构网站信息整理注:上表引用[1]Sinha, Sanju et al. “PERCEPTION predicts patient response and resistance to treatment using single-cell transcriptomics of their tumors.” Nature cancer vol. 5,6 (2024): 938-952.[2] Krishnamurthy, Savitri et al. “Fully Automated Artificial Intelligence Solution for Human Epidermal Growth Factor Receptor 2 Immunohistochemistry Scoring in Breast Cancer: A Multireader Study.” JCO precision oncology vol. 8 (2024): e2400353.[3] Pan, Xiaoxi et al. “The artificial intelligence-based model ANORAK improves histopathological grading of lung adenocarcinoma.” Nature cancer vol. 5,2 (2024): 347-363.[4] Dolezal, James M et al. “Uncertainty-informed deep learning models enable high-confidence predictions for digital histopathology.” Nature communications vol. 13,1 6572. 2 Nov. 2022.[5] https://medicine-uchicago-edu.libproxy1.nus.edu.sg/new-i3lung-project-to-fund-ai-tools-for-improving-treatment-and-outcomes-for-lung-cancer-patients/[6]https://keai-kaist-ac-kr.libproxy1.nus.edu.sg/projects/smc-digital-pathology-consortium/[7] https://www.itnonline.com/content/samsung-receives-fda-clearance-ai-algorithms-detect-lung-nodules-chest-x-rays[8]https://www.cancer.gov/news-events/press-releases/2024/ai-tool-matches-cancer-drugs-to-patient3.2 疾病领域聚焦疾病研究方面,AI应用聚焦于高发癌种:呼吸系统肿瘤居首(25.8%),与传统研究中NSCLC的高热度(16.1%)相契合;消化系统肿瘤(21.5%)与结直肠癌、胰腺癌等高突变负荷肿瘤关联紧密;乳腺及妇科肿瘤(13.5%)虽略低于传统研究占比(26.8%),但已有HER2表达评分等AI辅助系统临床落地,表明该领域AI应用仍处于加速渗透期。来源:根据AACR 2025大会网站信息,医药魔方的数据整理3.3 AI研究靶点特征靶点层面,AI研究对EGFR与PD‑L1的关注度最高(均15.4%),与第二、第三部分中统计的传统研究热点高度吻合,显示AI工具正用于增强这些经典靶点的表型识别与疗效预测能力。AI在HER2与KRAS(各12.8%)的应用中亦表现活跃,特别是在肺癌和结直肠癌中,深度学习模型被用于非侵入性预测基因突变状态[40,42]。此外,免疫相关靶点如CD3(10.3%)、MHC‑I(7.7%)的显著上升,标志着AI在肿瘤免疫微环境建模与抗原呈递机制探索中的扩展能力。少见靶点如DLL3(5.1%)的出现,提示AI正被用于罕见靶标的发现与亚群识别,支持新兴疗法如BiTE或CAR-T的早期开发[43]。表:TOP10靶点的AI研发案例来源:根据AACR 2025大会网站信息,PMC和 Pubmed文献库信息整理注:上表引用[1]Nguyen, Hung Song et al. “Predicting EGFR Mutation Status in Non-Small Cell Lung Cancer Using Artificial Intelligence: A Systematic Review and Meta-Analysis.” Academic radiology vol. 31,2 (2024): 660-683.[2] Yan, Fang et al. “Artificial intelligence-based assessment of PD-L1 expression in diffuse large B cell lymphoma.” NPJ precision oncology vol. 8,1 76. 27 Mar. 2024.[3] Dong, Yunyun et al. “Multi-channel multi-task deep learning for predicting EGFR and KRAS mutations of non-small cell lung cancer on CT images.” Quantitative imaging in medicine and surgery vol. 11,6 (2021): 2354-2375.[4] Krishnamurthy, Savitri et al. “Fully Automated Artificial Intelligence Solution for Human Epidermal Growth Factor Receptor 2 Immunohistochemistry Scoring in Breast Cancer: A Multireader Study.” JCO precision oncology vol. 8 (2024): e2400353.[5] Diao, James A et al. “Human-interpretable image features derived from densely mapped cancer pathology slides predict diverse molecular phenotypes.” Nature communications vol. 12,1 1613. 12 Mar. 2021.[6] Shao, Xiaoshan M et al. “High-Throughput Prediction of MHC Class I and II Neoantigens with MHCnuggets.” Cancer immunology research vol. 8,3 (2020): 396-408.[7] Frascarelli, Chiara et al. “Deep learning algorithm on H&E whole slide images to characterize TP53 alterations frequency and spatial distribution in breast cancer.” Computational and structural biotechnology journal vol. 23 4252-4259. 26 Nov. 2024.综上所述,AI在AACR 2025肿瘤研究中的布局已深入至主流机构、核心靶点与高发癌种的多个层面。当前的AI模型主要整合多组学数据、病理影像与临床信息,以实现复杂生物特征和分子表型的定量评估,对疾病特征进行分层预测,同时推动多模态数据交叉学习与智能协同。相较传统方法,AI赋能正使肿瘤的疗法和药物研发向“精准靶点发现+临床智能匹配”的方向升级,其在肿瘤的早期预测、患者筛选和精准治疗等环节的渗透将重塑未来肿瘤临床研究和药物开发模式。综上,全球肿瘤药物研发已步入“靶点+平台+智能”的三轴驱动时代。从实体瘤主导的靶点格局,到跨国药企与科研机构在新技术平台上的差异化尝试;从中国药企的国产替代到逐步崛起的创新靶点突破,再到AI技术对经典与新兴靶点的赋能重塑,2025年肿瘤研究生态已呈现出系统性变革特征。未来,靶点的“泛系统性”与“系统特异性”将共构临床策略主线;机构协作将加速从基础研究到转化应用的闭环效率;AI与多组学融合将成为肿瘤研究不可或缺的计算引擎。本研究报告希望为业界提供一份扎实的数据分析与趋势解读,助力各类主体在靶点选择、技术规划与国际协同中的前瞻决策与战略落位。张鸿宇浙江大学 生物与医药工程 在读博士中山大学 管理学硕士 法国格勒诺布尔高等经管学院 金融硕士(公派留学)个人简介浙江大学生物医药工程在读博士,专攻AI辅助精准肿瘤筛查,药物设计与开发;曾就职于平安集团、恒健金控和广发证券体系,从事生物科技和医疗卫生机构一级股债投融资业务,拥有AI、精准医疗与金融交叉复合背景,兼具前沿医学研发与资本运作双重视角,专注于AI赋能的精准医疗及新药研发,注重成果转化和市场应用。(微信号:18927501292)引用[1] https://www-aacr-org.libproxy1.nus.edu.sg/meeting/aacr-annual-meeting-2025/aacr-fast-facts/[2] Sung H, Ferlay J, Siegel RL, et al. “Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries.” CA: A Cancer Journal for Clinicians, 71(3):209–249, 2021.[3] Lauby-Secretan B, Scoccianti C, Loomis D, et al. “Body Fatness and Cancer — Viewpoint of the IARC Working Group.” New England Journal of Medicine, 375(8):794–798, 2016.[4] Jess T, Rungoe C, Peyrin-Biroulet L. “Risk of Colorectal Cancer in Patients With Ulcerative Colitis: A Meta-analysis of Population-based Cohort Studies.” Gastroenterology, 143(4): 860–868.e4, 2012.[5] Bardia A, Tolaney SM, Loirat D, et al. “Sacituzumab Govitecan–Hziy in Metastatic Triple-Negative Breast Cancer.” New England Journal of Medicine, 384(16):1529–1541, 2021.[6] https://www.fda.gov/news-events[7] U.S. Food and Drug Administration. “FDA Approves Sotorasib in Combination With Panitumumab for KRAS G12C–Mutated Metastatic Colorectal Cancer.” FDA News Release, May 2024. [8] Kandoth C, McLellan MD, Vandin F, et al. “Mutational Landscape and Significance Across 12 Major Cancer Types.” Nature, 502(7471):333–339, 2013.[9] Andre T, Shiu KK, Kim TW, et al. “Pembrolizumab versus Chemotherapy for MSI‑High/Mismatch Repair‑Deficient Metastatic Colorectal Cancer (KEYNOTE-177): Final Analysis of a Randomised, Open‑Label, Phase 3 Study.” Lancet Oncology, 22(7):931–942, 2021.[10] U.S. Food and Drug Administration. “FDA Approves Amivantamab and Lazertinib Combination for EGFR Mutated NSCLC.” FDA News Release, June 2024. [11] U.S. Food and Drug Administration. “FDA Grants Regular Approval to Pluvicto® for PSMA‑Positive Metastatic Castration‑Resistant Prostate Cancer.” FDA News Release, March 2024. [12] Onclive. “FDA Approves Zolbetuximab Plus Chemo for CLDN18.2+ Gastric or GEJ Adenocarcinoma.” Jul 2024.[13] U.S. FDA. “FDA Approves Sotorasib in Combination With Panitumumab for KRAS G12C–Mutated Metastatic Colorectal Cancer.” May 2024.[14] AACR. “Abstract CT019: Zoldonrasib May Elicit Objective Responses in KRAS G12D-Mutated NSCLC.” AACR Annual Meeting 2025.[15] U.S. FDA. “FDA Approves Amivantamab and Lazertinib Combination for EGFR Mutated NSCLC.” Jun 2024.[16] Arbour KC,Tawee T,Yaeger R,et al.CT019-Preliminary safety and antitumor activity of zoldonrasib(RMC-9805),an oral,RAS(ON)G12D-selective,tri-complex inhibitor in patients with KRAS G12D non-small cell lung cancer(NSCLC)from a phase 1 study in advanced solid tumors.Presented at:2025 AACR Annual Meeting;April 25-30,2025.[17] Schram, Alison M et al. “Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements.” Cancer discovery vol. 12,5 (2022): 1233-1247. [18] U.S. FDA. “FDA Grants Regular Approval to Pluvicto® for PSMA‑Positive Metastatic Castration‑Resistant Prostate Cancer.” Mar 2024.[19]https://www.globenewswire.com/news-release/2024/11/26/2987189/0/en/Roche-enters-into-a-definitive-agreement-to-acquire-Poseida-Therapeutics-including-cell-therapy-candidates-and-related-platform-technologies.html.[20] Cercek A. et al. “PD-1 Blockade in Mismatch Repair–Deficient Locally Advanced Rectal Cancer.” NEJM 386(25):2434–2442, 2022.[21] https://www.aacrmeetingnews.org/news/aacr-to-recognize-2025-scientific-achievement-award-recipients-during-annual-meeting.[22] ASCO. “Abstract 123: KRAS-Mutant NSCLC: Dual Pathway Inhibition Plus Immunotherapy.” ASCO 2023 Annual Meeting.[23] Novartis. “Novartis’ Pluvicto receives FDA approval for PSMA-positive mCRPC.” Novartis Press Release, March 2024.[24] Johnson & Johnson. “Rybrevant™ (amivantamab) plus Lazertinib receives FDA approval.” J&J Press Release, June 2024.[25] Pfizer. “FDA approves Braftovi (encorafenib) for BRAF V600E-mutant metastatic colorectal cancer.” Pfizer News, April 2024.[26] Arvinas. “Arvinas reports first patient dosed in Phase 1 trial of ARV-393 PROTAC.” Arvinas Press Release, February 2024.[27] Bristol Myers Squibb. “BMS advances CC‑199, an AR degrader, into preclinical development.” BMS News, January 2024.[28] Vertex Pharmaceuticals. “Vertex and CRISPR Therapeutics announce FDA approval of Casgevy, first CRISPR therapy.” Vertex Press Release, December 2023.[29] Hengrui Medicine. “Hengrui’s novel HER2-ADC SHR-A1811 designated as Breakthrough Therapy by NMPA.” Hengrui Annual Report 2023.[30] CStone Pharmaceuticals. “Preclinical proof-of-concept data for VBC108 dual-target ADC presented at AACR 2025.” CStone News, April 2025.[31] Junshi Biosciences. “Junshi’s toripalimab achieves NDA acceptance for NSCLC in China.” Junshi News, February 2024.[32] Innovent Biologics. “Innovent’s Cadonilimab (AK104) approved by NMPA for relapsed/refractory cervical cancer.” Innovent Press Release, May 2024.[33] Pharmcube (医药魔方). “2023 China Top20 Pharma R&D Landscape Report.” Pharmcube, January 2024.[34] Schumacher TN, Schreiber RD. “Neoantigens in cancer immunotherapy.” Science 348(6230):69–74, 2015.[35] Gillette MA, Satpathy S, Cao S, et al. “Clinical Proteomic Tumor Analysis Consortium (CPTAC) Investigates 2800 Putative Cancer Targets.” Cell, 179(2):340–355.e11, 2019.[36] Menden MP, Iorio F, Garnett M, et al. “Machine Learning for Integrative Analysis of Cancer Drug Sensitivity.” Nat. Commun. 9: 406, 2018.[37] Li B, Chen J, Wang X, et al. “CRISPR screening identifies novel immunotherapy targets in Chinese colorectal cancer cohort.” Nature Communications, 14: 3456, 2023.[38] Xie ZY, Chen H, Song Y, et al. “China’s integration into global oncology clinical trials: trends and challenges.” Lancet Oncology, 24(2): e75–e83, 2023.[39] National Cancer Institute. “F U.S. Food and Drug Administration. “FDA Approves Sotorasib in Combination With Panitumumab for KRAS G12C–Mutated Metastatic Colorectal Cancer.” FDA News Release, May 2024. ” NCI News Release, April 2024. [40] Krishnamurthy, Savitri et al. “Fully Automated Artificial Intelligence Solution for Human Epidermal Growth Factor Receptor 2 Immunohistochemistry Scoring in Breast Cancer: A Multireader Study.” JCO precision oncology vol. 8 (2024): e2400353.[41] https://keai-kaist-ac-kr.libproxy1.nus.edu.sg/projects/smc-digital-pathology-consortium/[42] Dong, Yunyun et al. “Multi-channel multi-task deep learning for predicting EGFR and KRAS mutations of non-small cell lung cancer on CT images.” Quantitative imaging in medicine and surgery vol. 11,6 (2021): 2354-2375.[43] Paz‐Ares L, et al. “Tarlatamab (AMG 757), a First‑in‑Class DLL3/CD3 Bispecific T‑Cell Engager, in Recurrent Small‑Cell Lung Cancer: An Open‑Label, Phase I Study.” Journal of Clinical Oncology, 41(15_suppl):8507, 2023.*封面图片来源:神笔PRO如果您认同文章中的观点、信息,或想进一步讨论,请与我们联系;也可加入动脉网行业社群,结交更多志同道合的好友。近期推荐声明:动脉网所刊载内容之知识产权为动脉网及相关权利人专属所有或持有。未经许可,禁止进行转载、摘编、复制及建立镜像等任何使用。文中如果涉及企业信息和数据,均由受访者向分析师提供并确认。动脉网,未来医疗服务平台
AACR会议细胞疗法免疫疗法疫苗
2023-07-06
The dynamics of the esophageal cancer market are predicted to grow owing to the increasing prevalence of cancer and the increase in research & development. In addition, the anticipated launch of emerging therapies will also propel the esophageal cancer market growth in the coming years.
LAS VEGAS, July 6, 2023 /PRNewswire/ -- DelveInsight's
Esophageal Cancer Market Insights report includes a comprehensive understanding of current treatment practices, esophageal cancer emerging drugs, market share of individual therapies, and current and forecasted market size from 2019 to 2032, segmented into 7MM [the United States, the EU-4 (Italy, Spain, France, and Germany), the United Kingdom, and Japan].
Key Takeaways from the Esophageal Cancer Market Report
As per DelveInsight analysis, the esophageal cancer market size in the 7MM was approximately
USD 1,000 million in 2022.
According to the assessment done by DelveInsight, the estimated total diagnosed incident esophageal cancer cases in the US were approximately
20K in 2022.
Globally, leading esophageal cancer companies such as
Zymeworks, Innovent Biologics, BeiGene, Apexigen, Shanghai Henlius Biotech, CStone Pharmaceuticals, Jiangsu HengRui Medicine Co., Ltd., AstraZeneca, Symphogen, Hoffmann-La Roche, Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Sinocelltech Ltd., Eli Lilly and Company, Ipsen, Jacobio Pharmaceuticals Co., Ltd., Shire, GlaxoSmithKline, Keythera Pharmaceuticals (Australia) Pty Ltd, Sunshine Lake Pharma Co., Ltd., Novartis Pharmaceuticals, Sanofi, Seagen Inc., Rapa Therapeutics LLC, Incyte Corporation, Atreca, Inc., Exelixis, Bayer, Lyell Immunopharma, Sichuan Baili Pharmaceutical, AP Biosciences., Leap Therapeutics, Inc., Adlai Nortye Biopharma Co., Ltd., Athenex, Inc., Pfizer, Genentech, Hangzhou Neoantigen Therapeutics, Janssen Pharmaceutical, Curis, Inc., Merck KGaA, Shenzhen Hornetcorn Bio-technology Company, LTD, MacroGenics, Bristol-Myers Squibb, Integral Molecular, CARTEXELL, EMD Serono, and others are developing novel esophageal cancer drugs that can be available in the esophageal cancer market in the coming years.
The promising esophageal cancer therapies in the pipeline include
Zanidatamab, Sintilimab, Tislelizumab, Sotigalimab (APX005M), and others.
Discover which therapies are expected to grab the major esophageal cancer market share @
Esophageal Cancer Market Report
Esophageal Cancer Overview
Esophageal cancer arises when cancer cells originate in the esophagus, a tube-like tissue that connects the throat and stomach. The esophagus transfers food from the mouth to the stomach. The cancer develops in the inner layer of the esophagus and can spread to other layers of the esophagus and other organs of the body. Typically, signs of esophageal cancer do not develop until the tumor has grown large enough to obstruct eating, swallowing, or digesting food. The most common symptom of esophageal cancer is difficulty swallowing, specifically a sense that food is trapped in the throat; in some cases, choking on food occurs. These symptoms worsen with time, with increasing discomfort while swallowing as the esophagus narrows due to cancer growth. Indications or symptoms of esophageal cancer are frequently used to diagnose it. Exams, testing, and a biopsy will be required to confirm the diagnosis; if cancer is found, additional tests will be performed to determine the extent.
Esophageal Cancer Epidemiology Segmentation
DelveInsight estimates that there were approximately
20K diagnosed incident cases of esophageal cancer in the US in 2022.
In 2022, as per the age-specific cases, the 65 and above segment accounted for the highest number of cases of esophageal cancer. In contrast, the <45 age group accounted for the least number of cases in the United States.
The esophageal cancer market report proffers epidemiological analysis for the study period 2019–2032 in the 7MM segmented into:
Total Esophageal Cancer Diagnosed Incident Cases
Esophageal Cancer Age-specific Cases
Esophageal Cancer Histology-specific Cases
Esophageal Cancer Gender-specific Cases
Esophageal Cancer Mutation-specific Cases
Esophageal Cancer Stage-specific Cases
Esophageal Cancer Line-wise Treated Cases
Esophageal Cancer Treatment Market
Everyone with esophageal cancer is treated in some way. The best course of therapy for each patient is determined by a number of criteria, including personal preferences, cancer stage, and overall health. Many medical specialists work together on a daily basis in cancer care to create a patient's comprehensive treatment plan, which includes a variety of treatments. Esophagectomy is the primary esophageal cancer treatment for early-stage esophageal cancer, while its precise role in superficial (T1A) malignancies remains unknown, owing to the introduction of endoscopic mucosal therapy. A multimodal strategy of neoadjuvant chemotherapy or combination chemoradiotherapy (CRT) followed by surgery is strongly recommended for treating locally advanced malignancies.
Preoperative evaluation is the cornerstone of modern esophageal cancer management. The precision and specificity of the clinical staging evaluation will be dependent on the tumor board's recommendations on the use of multimodal therapy, thus preoperative staging accuracy is crucial. The standardized evaluation of a patient undergoing curative treatment for early-stage or advanced esophageal cancer includes upper endoscopy, high-resolution contrast CT scan, FDG-PET scan, and EUS.
For a tumor that has not migrated beyond the esophagus and lymph nodes, doctors often recommend a combination of radiation treatment, chemotherapy, and surgery. Locally advanced esophageal cancer is typically treated with radiation therapy, chemotherapy, and surgery. Radiation treatment and chemotherapy are frequently combined in "chemoradiotherapy." Radiation therapy, chemotherapy, and other drug-based therapies are frequently used to treat metastatic esophageal cancer.
To know more about esophageal cancer treatment, visit @
Esophageal Cancer Treatment Drugs
Key Esophageal Cancer Therapies and Companies
Zanidatamab: Zymeworks
Sintilimab: Innovent Biologics
Tislelizumab: BeiGene
Sotigalimab (APX005M): Apexigen
Nivolumab: Bristol-Myers Squibb
Pembrolizumab: Merck Sharp & Dohme
Learn more about the FDA-approved drugs for esophageal cancer @
Drugs for Esophageal Cancer Treatment
Esophageal Cancer Market Dynamics
The esophageal cancer market is expected to change in the coming years owing to
expanding disease awareness and
increased healthcare spending around the world, which will increase the size of the esophageal cancer market. The esophageal cancer
companies and academics are working to assess challenges and seek opportunities that could influence esophageal cancer R&D. The therapies under development are focused on novel approaches to treat/improve the disease condition. Esophageal cancer companies are involved in developing esophageal cancer therapies. Moreover, the
anticipated introduction of emerging therapies with improved efficacy and a further
improvement in the diagnosis rate are expected to drive the growth of the esophageal cancer market in the 7MM.
However, several factors are impeding the growth of the esophageal cancer market. The
cost of newly approved drugs is typically exorbitant, so people avoid correct therapy or
opt for off-label and low-priced medications. It has an impact on market access for newly released drugs, and reimbursement is critical.
Market access and reimbursement options might vary depending on regulatory status, target population size, care environment, unmet requirements, the volume of extra benefit claims, and costs. Furthermore, the esophageal cancer market growth may be offset by
failures and discontinuation of emerging therapies and a
shortage of healthcare specialists. In addition, the
undiagnosed, unreported cases and the unawareness about the disease may also impact the esophageal cancer market growth.
Scope of the
Esophageal Cancer
Market Report
Therapeutic Assessment: Esophageal Cancer current marketed and emerging therapies
Esophageal Cancer
Market Dynamics: Attribute Analysis of Emerging Esophageal Cancer Drugs
Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
Unmet Needs, KOL's Views, Analyst's Views, Esophageal Cancer Market Access and Reimbursement
Discover more about esophageal cancer drugs in development @
Esophageal Cancer Clinical Trials
Table of Contents
Related Reports
Esophageal Cancer Pipeline
Esophageal Cancer Pipeline Insight – 2023 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key esophageal cancer companies, including
BeiGene, AstraZeneca, CSPC ZhongQi Pharmaceutical Technology, Amgen, Jiangsu Hengrui Medicine, Oncolys Biopharma, Pfizer, Novartis, Luye Pharma, Eli Lilly and Company, ImmunoFrontier, Inc, Adaptimmune, Innovent Biologics (Suzhou) Co. Ltd., Genentech, Janssen Pharmaceuticals, Merck KGaA, Shenzhen Hornetcorn Biotechnology, Apexigen, Inc., Highlight Therapeutics, EMD Serono, HaiHe Biopharma, Lumicell, Jacobio Pharmaceuticals Co., Ltd., TESARO, Ascentage Pharma Group Inc., Adlai Nortye, Seagen, among others.
Gastro-Esophageal Junction Neuroendocrine Tumor (GEJ-NET) Market
Gastro-Esophageal Junction Neuroendocrine Tumor (GEJ-NET) Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key GEJ-NET companies, including
Vedanta Biosciences, Inc., Bristol-Myers Squibb, Incyte Corporation, Xencor, Inc., ICON plc, Adaptimmune, Medicenna Therapeutics, Inc., Guangdong Zhongsheng Pharmaceutical Co., Ltd., among others.
Esophageal Squamous Cell Carcinoma Pipeline
Esophageal Squamous Cell Carcinoma Pipeline Insight – 2023 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key esophageal squamous cell carcinoma companies, including
BeiGene, AstraZeneca, Ipsen, Shire, Sanofi, Seagen, among others.
Esophageal Squamous Carcinoma Market
Esophageal Squamous Carcinoma Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key esophageal squamous carcinoma companies, including
BeiGene, AstraZeneca, Ipsen, Shire, Sanofi, Seagen, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve
.
Expand your knowledge and gain valuable insights into personalized cancer care through
DelveInsight's Conference Coverage Services
Explore some of the conference coverage related case studies:
Conference Coverage in Various Oncology Conferences
Conference Coverage Intelligence of Asthma and COPD assets across the Respiratory Therapy Area
Contact Us
Shruti Thakur
[email protected]
+1(919)321-6187
Logo:
SOURCE DelveInsight Business Research, LLP
放射疗法ASCO会议
2022-10-05
前 言 PD1、PDL1、CTLA4等免疫检查点蛋白抑制剂(ICIs)的成功, 使科学家们投入到肿瘤免疫治疗药物的开发热潮中。最近一些药物已进入临床试验,其中除了靶向表面蛋白的抗体外,不乏一些具有免疫刺激效应的小分子药物(见表1)。口服小分子药物克服了抗体药物不良反应多、半衰期长、输注给药不便等局限,为药物发现开辟了一个全新的领域。此外,肿瘤免疫小分子药物与免疫检查点抑制剂具有协同作用,两者联合用药的方案也得到了越来越多的批准。今年8月发表的Nature reviews盘点了肿瘤免疫治疗中的小分子药物及其应用发展 [1]。表1:肿瘤免疫治疗部分小分子药物作者 | 梨子君靶向细胞生长和肿瘤代谢的小分子抑制类药物01靶向促肿瘤通路癌症的两个固有的特征是失去细胞稳态周期控制和增加凋亡抵抗。另外,肿瘤持续的生长需要一个有利的微环境,并由促进血管生成、炎症和组织重塑的通路驱动。进展中的肿瘤能量需求不断增加,因此癌细胞重新调整其代谢途径,导致营养和代谢物浓度的显著变化,这些因素共同参与了肿瘤微环境(TME)的塑造 [2]。目前已经开发了各种靶向肿瘤细胞固有特征和TME途径的小分子药物。1. 细胞生长抑制剂细胞生长抑制类药物的疗效部分依赖抗肿瘤免疫反应的间接刺激,该类药物诱导的免疫原性细胞死亡(ICD)可以触发抗肿瘤T细胞应答的激活(图1)。在进入TME后,T细胞会遇到抑制信号而功能减弱,因此常常联合化疗和免疫检查点抗体。然而,化疗往往与淋巴减少症相关,会抵消诱导的适应性免疫反应。因此,联合具有选择性的靶向药物可能是更好的用药方案。在BRAF(V600E)转移性黑色素瘤中,BRAF抑制剂与MEK抑制剂联合应用是治疗的标准方法,之后进展的患者常用PD-1抗体进行二线治疗。这使得研究人员探索BRAF/MEK抑制剂与ICIs的三联治疗。两项I期研究表明,BRAF/MEK抑制可导致ICD,产生促炎信号,增加CD8+T细胞浸润,提高MHC I表达水平,使肿瘤细胞对T细胞介导的攻击更敏感。在这种情况下,同时给予ICI会以协同方式维持T细胞活化。此外,小分子抗肿瘤药物的影响可能不止于诱导ICD。MEK抑制可在多个水平上直接调节免疫浸润的性质,例如通过减少CD4+调节性T细胞(Treg细胞)、髓源性抑制细胞(MDSC)和M2型肿瘤相关巨噬细胞(TAM),导致TME中CD8+/Treg淋巴细胞和M1/M2巨噬细胞比值增加。一项II期疗效试验显示,与治疗组合达布雷尼布(BRAF抑制剂)和曲美替尼(MEK抑制剂)相比,加上帕博利珠单抗(PD1 抗体)的三联疗法延长了患者的无进展生存期。IMspire150 III期研究也也观察到同样的效果,这项研究评估了阿替利珠单抗(PDL1抗体)、维莫非尼(BRAF抑制剂)和考比替尼(MEK抑制剂)的联合,并于2020年7月获FDA批准用于治疗BRAF(V600E)突变型黑色素瘤。图1:免疫原性细胞死亡调节T细胞活性2. VEGF–VEGFR 抑制剂血管内皮生长因子(VEGF)是一种刺激血管生成的信号蛋白,能抑制抗原提呈、招募免疫抑制细胞亚群、破坏抗肿瘤T细胞反应。高血管化肿瘤(如肾细胞癌)对VEGF–VEFGR通路抑制剂介导的血管生成阻断特别敏感。ICIs与这种药物的结合具有协同效益。2018年12月FDA首次批准贝伐单抗(VEGF 抗体)与阿替利珠单抗联合用于晚期非小细胞肺癌的一线治疗。2019年,阿西替尼成为首个FDA注册的小分子VEGFR酪氨酸激酶抑制剂,与帕博利珠单抗或阿维单抗(PDL1 抗体)联合用于晚期肾细胞癌的一线治疗。两种方案均优于迄今为止针对该适应症的标准方案——广谱激酶抑制剂舒尼替的单药治疗。目前,在其他实体肿瘤(包括黑素瘤、头颈癌、肝细胞癌、结直肠癌、胃癌和妇科癌)中,已有超过100项类似方案的试验启动,总体结果有望获得FDA批准 [3] 。3. 细胞因子、趋化因子通路阻断剂细胞因子超家族在细胞交流中起着至关重要的作用,特别是在免疫系统中。干扰素-α (IFNα)和白细胞介素-2 (IL-2)对T细胞增殖和效应功能有强烈刺激作用,成为第一批用于癌症免疫治疗的细胞因子。然而,在大多数情况下,这些细胞因子治疗无法重现临床前模型的抗肿瘤疗效。迄今为止,阻断促癌炎症过程中的细胞因子通路和/或抑制T细胞免疫的治疗方法,如IL-1、IL-4、IL-6、IL-8、IL-10、TGFβ和CSF1,在临床研究中也没有表现出一致的疗效。尽管其中一些药物与ICIs和/或细胞抑制模式的组合可能被证明对癌症患者有效,但TME中细胞因子网络的复杂性使得单一通路抑制的抗癌效果削弱。从免疫治疗的角度来看,抑制CSF1-CSF1R通路可能是一种更有前途的策略。高水平的CSF1会促进微环境中TAMs和MDSCs的浸润和扩张,在临床前模型中抑制该通路能减少髓细胞浸润并增加CD8+ T细胞,从而与ICIs协同作用。因此,该途径的多种抗体和小分子抑制剂或作为单一药物,或与细胞抑制药物或ICIs联合使用(图2)。图2:CSF1R抑制剂结构2019年,FDA批准小分子CSF1R-TK抑制剂培西达替尼用于治疗腱鞘巨细胞瘤 (TGCT)。ARRY-382是一种选择性更强的CSF1R抑制剂,在一项II期临床试验中与pembrolizumab联合治疗晚期实体肿瘤,但辉瑞公司最近发布的研究报告显示该方案对患者没有任何有益的效果。相比其他人类激酶,Vimseltinib对3类RTKs具有超过100倍的选择性。2022年9月12日,生物制药公司Deciphera Pharmaceuticals Inc.在ESMO上公布了Vimseltinib治疗腱鞘巨细胞瘤I/II期试验的最新数据,显示31名患者都表现出临床获益,A组的客观反应率为53%,B组为46%。另一种选择性CSF1R抑制剂BLZ945是一种脑穿透剂,目前正在进行I/II期临床试验,作为单药或与斯帕巴他珠单抗(PD1 抗体)联合用于治疗晚期实体肿瘤及中枢神经系统癌症。初步数据显示对胶质母细胞瘤患者具有抗肿瘤活性和可接受的安全性。02 靶向代谢途径肿瘤生长过程中各种代谢物的积累促进TME的形成,驱动这一过程的几种酶促蛋白可以被靶向调节,目前研究与小分子开发较为深入的主要为以下三种途径。1. 谷氨酰胺和精氨酸代谢抑制剂癌细胞通过促进糖酵解和谷氨酰胺分解来满足对能量和生物合成前体的高需求。值得注意的是,TME中不止有肿瘤细胞,T细胞、髓细胞以及其他基质细胞之间对葡萄糖、谷氨酰胺和其他营养物质存在激烈的竞争。尽管临床前模型中,用小分子抑制这些代谢通路可导致肿瘤生长减少和免疫细胞浸润重编程,但在临床试验中仍缺乏具体证据。如著名的口服谷氨酰胺酶抑制剂CB839,基于临床前的成功数据进行了多项临床研究。尽管如此,由于在两项针对肾细胞癌和非小细胞肺癌的试验中没有临床效益,Calithera Biosciences最近停止了该化合物的开发。肿瘤、T细胞和MDSCs都依赖于精氨酸,从而进一步增加了这种氨基酸在TME中的需求。目前,一些小分子精氨酸酶抑制剂已经被开发出来,其中一种是硼酸基化合物INCB001158,已被Incyte纳入五项早期临床试验,在这些研究中,该药单独或与ICIs联合试验。初步试验报告表明,INCB001158的耐受性良好,临床疗效的信息有待全面评估发表。但截至最近,INCB001158已不再列在Incyte的投资组合中,这表明其临床开发已经停止。另外一种抑制剂是Calithera公司开发的CB-280,正在对囊性纤维化患者进行安全性评估。除上两者外,目前没有进一步的精氨酸酶抑制剂在临床试验中。2. 腺苷途径阻断剂腺苷是一种强大的免疫抑制剂,许多肿瘤产生高浓度的腺苷 [4]。其免疫抑制特性是由两个G蛋白偶联受体(GPCRs)介导的,即高亲和力的A2aR和低亲和力的A2bR(图3)。腺苷途径阻断剂imaradenant、ciforadenant和inupadenant特异性抑制A2aR,从2015年开始进行I/II期肿瘤试验,但迄今尚未报告临床疗效的明确证据。目前科研人员的注意力转向双A2aR/A2bR抑制剂。一个例子是etrumadenant,两种受体的拮抗剂,其可联合zimberelimab(PD1 抗体)和多西他赛(多烯紫杉醇)治疗转移性去势抵抗性前列腺癌患者。此外,在结直肠癌中,etrumadenant联合FOLFOX化疗作为三线治疗显示出有临床意义的疗效。另外,考虑到TME中的高腺苷浓度,高亲和力A2aR可能会被永久打开,其他研究人员正在开发低亲和度A2bR的阻断剂。Teon Therapeutics已开始开发TT-702,这是一种选择性A2bR拮抗剂,对免疫细胞和癌细胞都有活性。Taros Therapeutics也在开发A2bR选择性分子。在TME中,腺苷由外胚层酶CD73催化AMP生成,因此CD73成为该途径另一重要靶点。除了几种Ab类药物外,还有AB680和OP5244/ORIC-533两个小分子正在研究。此外,腺苷可以通过各种额外的外泌酶产生,如CD38、CD39、CD203a/外泌核苷酸磷酸磷酸酶/磷酸二酯酶家族成员1、碱性磷酸酶和前列腺酸性肽酶(图3)。目前,针对CD38和CD39的临床抗体已经存在,未来可能会产生更多针对这组外胚层酶的小分子药物。该途径之后的方向可以聚焦在正确靶点组合的选择上。图3:腺苷途径相关通路3. 犬尿氨酸途径抑制剂肿瘤细胞和间质细胞可以在IDO1和TDO两种酶介导的反应中分解色氨酸,从而产生犬尿氨酸 [5]。由此导致的色氨酸缺乏和TME中犬尿氨酸的积累都会抑制免疫反应。在与芳香烃受体(AHR)结合后,犬尿氨酸可以抑制效应T细胞,促进 CD4+ Treg细胞和MDCSs的发展(图4)。临床实验表明,该途径的阻断在IDO1和犬尿氨酸水平高的患者中更具价值。林罗多司他是一种更有效的、不可逆的IDO1抑制剂, 耐受性良好,目前正与纳武单抗联合用于黑色素瘤患者的III期临床试验中。但是,其他色氨酸降解酶TDO和IDO2的代偿性表达可能使肿瘤对IDO1抑制不敏感。此外,IL-4诱导-1(IL4i1)最近被确认为一种额外的色氨酸分解酶,由肿瘤和髓系细胞分泌到TME(图4)。因此,可能需要同时抑制上述几个靶点,从而提高临床试验中的疗效。最近已报道IDO1/TDO双抑制剂(如RG-70099和M4112)。另外,两种AHR阻断剂(BAY2416964和IK175)已进入实体肿瘤的临床试验。图4:犬尿氨酸途径介导的抗肿瘤免疫靶向固有免疫和免疫检查点的小分子药物03靶向固有免疫通路最早时曾在肿瘤内注射Coley疫苗和卡介苗(BCG)治疗癌症,尽管当时并不清楚作用机制,但现在我们知道,注射细菌提取物会触发多种固有免疫途径,从而介导肿瘤细胞的杀伤。1. TLR 激动剂Toll样受体(TLR)是一个1型跨膜蛋白家族,通过触发固有反应和介导适应性反应的激活,在协调病原体免疫方面起着关键作用 [2]。病原体相关分子模式(PAMPs)作为TLR配体,促进新表位的交叉呈递,在引流淋巴组织中激活树突状细胞。到目前为止,三种TLR激动剂已被批准用于肿瘤免疫治疗:用于膀胱癌局部免疫治疗的TLR2/TLR4刺激物卡介苗、用于局部治疗选定上皮病变的小分子TLR7/TLR8配体咪喹莫特,以及单磷酸脂A(MPL),一种TLR4配体,用作预防性人乳头瘤病毒(HPV)疫苗Cervarix95的佐剂。许多TLR激动剂已经在临床试验中进行了研究,其中大多数靶向内质体TLR。尽管在临床前肿瘤模型中使用各种TLR激动剂是有效的,但临床试验的结果一直令人失望,这主要有三个主要原因。一是TLR在许多不同的细胞类型上表达,包括不同程度上的肿瘤细胞,使得TLR激动剂的体内作用机制难以预测;二是TLR的表达模式和参与的生物学反应在小鼠和人类之间有很大差异;三是TLR激动剂全身给药有诱导全身免疫激活的高风险,与细胞因子释放综合征等严重不良事件相关。但TLR7激动剂LHC165克服了一些困难。它被吸附到氢氧化铝上,使其从注射部位缓慢释放,激活局部受限的免疫。据报道,在晚期实体瘤患者中首次出现临床抗肿瘤活性,包括作为单药或与sparvatigumab(PD1抗体)联合使用,尤其是对于基线时免疫微环境活跃的患者。为了降低全身给药时的毒性风险,目前正在探索通过抗体-药物结合物(ADC)靶向给予TLR激动剂。例如,SBT6050将强效TLR8激动剂与抗HER2抗体结合起来治疗HER2表达实体肿瘤。2. STING激动剂除TLR外,其他“危险”传感器主要响应细胞内源性分子信号(DAMPs),从而为激活先天免疫和适应性免疫提供额外途径。干扰素基因刺激物(STING)通路对非凋亡细胞死亡释放的DNA片段作出反应。这些DNA片段与细胞溶质酶cGMP–AMP合成酶(cGAS)的结合,导致环状二核苷酸cGAMP的产生,并激活内质网相关蛋白STING。随后,STING与TANK结合激酶1(TBK1)结合,然后该复合物转移到核周区域并激活下游介质,特别是转录因子干扰素调节因子3(IRF3)(图1)[3]。临床上第一代STING激动剂主要基于环状二核苷酸(CDN),如ADU-S100和MK-1454。但这两种化合物远远落后于预期,临床开发已被终止。与TLR激动剂类似,这些化合物的一个关键限制是需要肿瘤内注射。第一种全身给药的STING激动剂SB 11285和SNX281正在与PD1/PDL1抗体联合进行临床评估。此外,含有氨基苯并咪唑框架的非CDN基STING拮抗剂在小鼠中的药代动力学和全身活性得到改善。GSK3745417作为单一疗法以及与帕博利珠单抗联合治疗晚期实体瘤正在进行I期临床试验。当然,STING激活仍存在一些不确定性。过度刺激cGAS–STING途径有诱发细胞因子风暴的风险,最近的临床前数据表明,STING激活可通过触发应激和促凋亡途径诱导T细胞死亡。3. ENPP1、TREX1和PARP7抑制剂鉴于直接激活STING所面临的挑战,最近的策略侧重于以间接方式刺激该通路,如针对STING负调控因子ENPP1。ENPP1降解免疫刺激代谢物cGAMP和ATP,从而参与免疫抑制腺苷的生成(图1)。抑制ENPP1可通过增加cGAMP水平导致STING激活,同时减少对腺苷通路的刺激。一些口服ENPP1抑制剂已经进行了临床前研究,如MV626、SR-8314及其后续分子SR-8541。到目前为止还没有开始临床试验。进一步激活STING途径的方法是抑制3 ' 核酸修复外切酶1 (TREX1),它负责降解肿瘤来源的DNA。抑制TREX1会导致胞质DNA的积累和STING通路的激活。小分子TREX1抑制剂的首批实例最近由Constellation Pharmaceutical和Venenum Biodesign LLC公布。多聚ADP核糖聚合酶7(PARP7)在吸烟相关性强的癌症中过度表达,通过与TBK1相互作用负调节I型干扰素反应。因此,它也作为STING途径的抑制剂(图1)。RBN-2397是该酶的一种有效特异性阻断剂,最近已进入I期安全试验。图1:STING相关通路04小分子免疫检查点抑制剂(ICIs)尽管免疫检查点抗体已成为免疫治疗的金标准,但小分子药物相对于抗体药物的潜在优势引发了干扰PDL1–PD1轴的药物化学研究。另外,随着对T细胞内信号的不断深入研究,发现了T细胞受体(TCR)参与下游的几个负反馈回路,这些回路可能增强抗肿瘤T细胞免疫(图2)。主要的成药靶点包括造血祖激酶1(HPK1或MAP4K1)、二酰基甘油激酶(DGKα和DGKζ)、酪氨酸蛋白磷酸酶非受体型(PTPN6和 PTPN22)、E3泛素蛋白连接酶CBL-B等。图2:参与免疫受体信号传递的胞内靶点1. PDL1–PD1小分子抑制剂长期以来,PDL1-PD1的相互作用被认为不受小分子的抑制。尽管如此,第一批口服小分子药物CA-170(图3)和GS-4224已进入临床。CA-170来源于氨基酸丝氨酸-天冬酰胺-苏氨酸序列,该序列是由PD1初级序列的基序得到的。据报道,该化合物可靶向PDL1和VISTA。VISTA是一种检查点调节剂,在髓细胞上高度表达。核磁共振光谱显示CA-170在不阻止络合物形成的情况下产生缺陷的PD1-PDL1三元络合物,且在小鼠体内的口服生物利用度为50%。而GS-4224的安全性研究已被终止。另一种分子ARB-272572(图3)在亚纳摩尔浓度下可干扰PDL1功能,并在可移植的同源结直肠癌小鼠模型和肝炎病毒感染模型中显示出活性。一种相关的联苯分子INCB086550(图3)正在实体肿瘤患者中进行I/II期临床研究。口服这种化合物可抑制过表达PDL1的MC38肿瘤,其效力与阿替利珠单抗相当。这些化合物口服似乎增加了T细胞肿瘤浸润,不仅通过与PDL1结合,还通过诱导PD1的细胞内化。第一批小分子ICIs进入临床是十分重要的一步,但这些化合物在哪些方面可以达到临床上抗体的最佳状态,仍有待观察。图3:检查点小分子抑制剂2. MAP4K1抑制剂MAP4K1是一种丝氨酸/苏氨酸激酶,仅在造血细胞类型中表达 [4]。通过其富含脯氨酸的结构域,MAP4K1能够与造血细胞中的各种配体结合,MAP4K1的功能已经在TCR信号环境中得到了详细研究。TCR刺激后,MAP4K1在酪氨酸381上磷酸化,随后,MAP4K1被募集到TCR信号复合体中,通过其丝氨酸/苏氨酸激酶功能诱导该复合体解离,起到负调控作用。最近,一些论文揭示MAP4K1是小分子干预的合适靶点。最早发表的MAP4K1抑制剂之一是ZYF0033。据报道,该化合物可以抑制MAP4K1,影响近端生物标志物pSLP76,在4T1同基因小鼠模型中剂量依赖性的抑制肿瘤生长。BAY-405是另一个最近公布的MAP4K1抑制剂。该分子可以抑制SLP76的磷酸化,具有亚微摩尔的效力,并显示出良好的激酶选择性。体外功能分析显示BAY-405可增强人细胞毒性T细胞中细胞因子的活化和产生。BAY-405在同基因EMT6乳腺和B16-OVA黑色素瘤小鼠模型中显示了单药疗效,在B16-OVA模型中与抗PDL1 Ab联合使用时也有疗效。一些MAP4K1抑制剂已在实体肿瘤中进行了试验,例如NDI-101150/NMBS-2,具有很强的细胞效力,对所有MAP4K家族成员具有>100×的选择性。NDI-101150随后在一组同基因体内疗效模型中进行了评估,12个模型中有4个被观察到单药治疗肿瘤生长减少。重要的是,在EMT6同基因乳腺肿瘤模型中观察到肿瘤完全减少。联合抗PD1 Ab可以恢复衰竭T细胞的细胞因子分泌,并在CT26肿瘤模型中抑制肿瘤生长。此外,MAP4K1抑制剂CFI-402411的I/II期试验已开始评估单药和与帕博利珠单抗联合使用的耐受性。另外三种MAP4K1小分子抑制剂BGB-15025、PRJ1-3024和PF-07265028也已进入临床研究。3. DGKα和DGKζ抑制剂二酰基甘油(DAG)是磷脂酶Cγ1在TCR作用时产生的第二信使,触发信号级联,在T细胞发育和功能中起重要作用。DGKs家族催化DAG磷酸化为磷脂酸,从而调节DAG介导的信号。其中,DGKα和DGKζ在T细胞中显著表达 。两种DGKs的表达和活性在TCR刺激下增强,而持续表达与肿瘤浸润T细胞的低反应性相关(图2)。DGKα小分子抑制剂R59022, R59949和利坦色林已经被发现几十年。这些第一代DGK抑制剂的体外实验已证明通过药理抑制DGKs增强T细胞活性的概念,以及DGK抑制与PD1 阻断的联合作用。在小鼠中,用利坦色林治疗可以克服对PD1阻断的耐药性。百时美施贵宝最近公布的一项专利申请声称双DGKα/DGKζ抑制剂是T细胞激活剂。其中一种抑制剂对DGKα的抑制作用低于1毫摩尔。目前开发临床DGKs抑制剂的一个关键顾虑是脱靶效应。4. CBL-B抑制剂泛素化,即泛素部分附着在蛋白质赖氨酸残基上,是蛋白质的翻译后修饰,参与调节多种信号通路和细胞过程。CBL-B是一个RING家族E3泛素连接酶,作为TCR激活的负调控下游发挥作用 [5] 。其活性被CD28共刺激抑制,并被PD1和CTLA4接合诱导。最初的CBL-B靶向研究集中在非小分子方法上,包括干扰CBL-B功能的细胞渗透性肽和通过小干扰RNA调控CBLB基因表达或靶向基因组编辑。2016年,Progenra提出了一系列已知的小分子CBL-B抑制剂,能够通过抑制TAM受体的泛素化激活T细胞和NK细胞活性,但缺乏后续研究。目前已知的唯一CBL-B抑制剂,由Nurix开发,可增强体外T细胞的反应性,以及可移植的同基因小鼠模型中T细胞依赖性肿瘤的消退。化合物NX-1607在晚期恶性肿瘤中的安全性试验最近已经启动。泛素系统的复杂性和对底物特异性的未知提高了选择性小分子CBL-B抑制剂的发现门槛。5. 磷酸酶几种磷酸酶(包括SHP1、SHP2和PTPN22等)介导T细胞信号转导的负调控。SHP099是一种选择性的、口服的SHP2抑制剂,在免疫缺陷小鼠中显示了对人类肿瘤异种移植的抗肿瘤效果。另一种SHP2抑制剂RMC-4550的试验显示,其在CT26和EMT6同基因肿瘤模型中具有中等的抗肿瘤效果,同时对PDL1和CTLA4抗体也具有补充作用。几种SHP2抑制剂正在与ICI Abs联合进行临床评估。TNO155与斯巴达珠单抗联合、RMC-4630与帕博利珠单抗联合都处于I期临床试验中。结 语胞生长抑制剂、VEGF–VEGFR 抑制剂和细胞因子、趋化因子抑制剂等传统小分子药与ICIs联合应用具有更大的潜力。针对代谢途径的阻断剂可以改变肿瘤或TME中其他免疫细胞的行为,从而激活对肿瘤的杀伤反应。科研人员正在全力扩大肿瘤免疫治疗的方案,其中小分子药物发挥着越来越重要的作用(图4)。尽管一些靶向促肿瘤途径的小分子已被批准作为PD1-PDL1抗体的伴侣,但未来几年将探索小分子是否也可以应用于临床,直接刺激T细胞和/或先天抗肿瘤免疫。图4:TME造成的免疫调节障碍与解决者小分子[1] Offringa R, Kötzner L, Huck B, Urbahns K. The expanding role for small molecules in immuno-oncology [published online ahead of print, 2022 Aug 18]. Nat Rev Drug Discov. 2022;10.1038/s41573-022-00538-9. doi:10.1038/s41573-022-00538-9.[2] Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12(1):31-46. doi:10.1158/2159-8290.CD-21-1059.[3] Hack SP, Zhu AX, Wang Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front Immunol. 2020;11:598877. Published 2020 Nov 5. doi:10.3389/fimmu.2020.598877.[4] Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology [published correction appears in Nat Rev Clin Oncol. 2020 Jul 17;:]. Nat Rev Clin Oncol. 2020;17(10):611-629. doi:10.1038/s41571-020-0382-2.[5] Opitz CA, Somarribas Patterson LF, Mohapatra SR, et al. The therapeutic potential of targeting tryptophan catabolism in cancer. Br J Cancer. 2020;122(1):30-44. doi:10.1038/s41416-019-0664-6.扫码加入BiG生物创新社读者交流群,分享、交流纯粹的行业知识,非诚勿扰!BiGScientific Driven, Making Impact!创新生态丨医药论坛丨行业分析媒体公关丨BiG Webinar联系我们商务:Max 18662346610媒体:Kathy 17621909690
小分子药物免疫疗法抗体抗体药物偶联物疫苗
100 项与 帕博利珠单抗生物类似药(Sandoz) 相关的药物交易
登录后查看更多信息
外链
| KEGG | Wiki | ATC | Drug Bank |
|---|---|---|---|
| - | - | - |
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 转移性非小细胞肺癌 | 临床3期 | 美国 | 2024-04-29 | |
| 转移性非小细胞肺癌 | 临床3期 | 日本 | 2024-04-29 | |
| 转移性非小细胞肺癌 | 临床3期 | 波斯尼亚和黑塞哥维那 | 2024-04-29 | |
| 转移性非小细胞肺癌 | 临床3期 | 巴西 | 2024-04-29 | |
| 转移性非小细胞肺癌 | 临床3期 | 德国 | 2024-04-29 | |
| 转移性非小细胞肺癌 | 临床3期 | 印度 | 2024-04-29 | |
| 转移性非小细胞肺癌 | 临床3期 | 马来西亚 | 2024-04-29 | |
| 转移性非小细胞肺癌 | 临床3期 | 菲律宾 | 2024-04-29 | |
| 转移性非小细胞肺癌 | 临床3期 | 罗马尼亚 | 2024-04-29 | |
| 转移性非小细胞肺癌 | 临床3期 | 塞尔维亚 | 2024-04-29 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
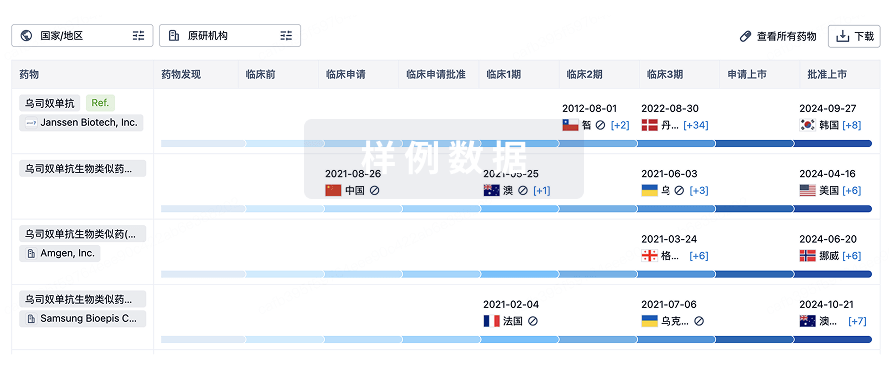
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

