预约演示
更新于:2025-11-06
Donidalorsen
更新于:2025-11-06
概要
基本信息
非在研机构- |
最高研发阶段批准上市 |
首次获批日期 美国 (2025-08-21), |
最高研发阶段(中国)- |
特殊审评孤儿药 (美国)、孤儿药 (欧盟)、孤儿药 (澳大利亚) |
登录后查看时间轴
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
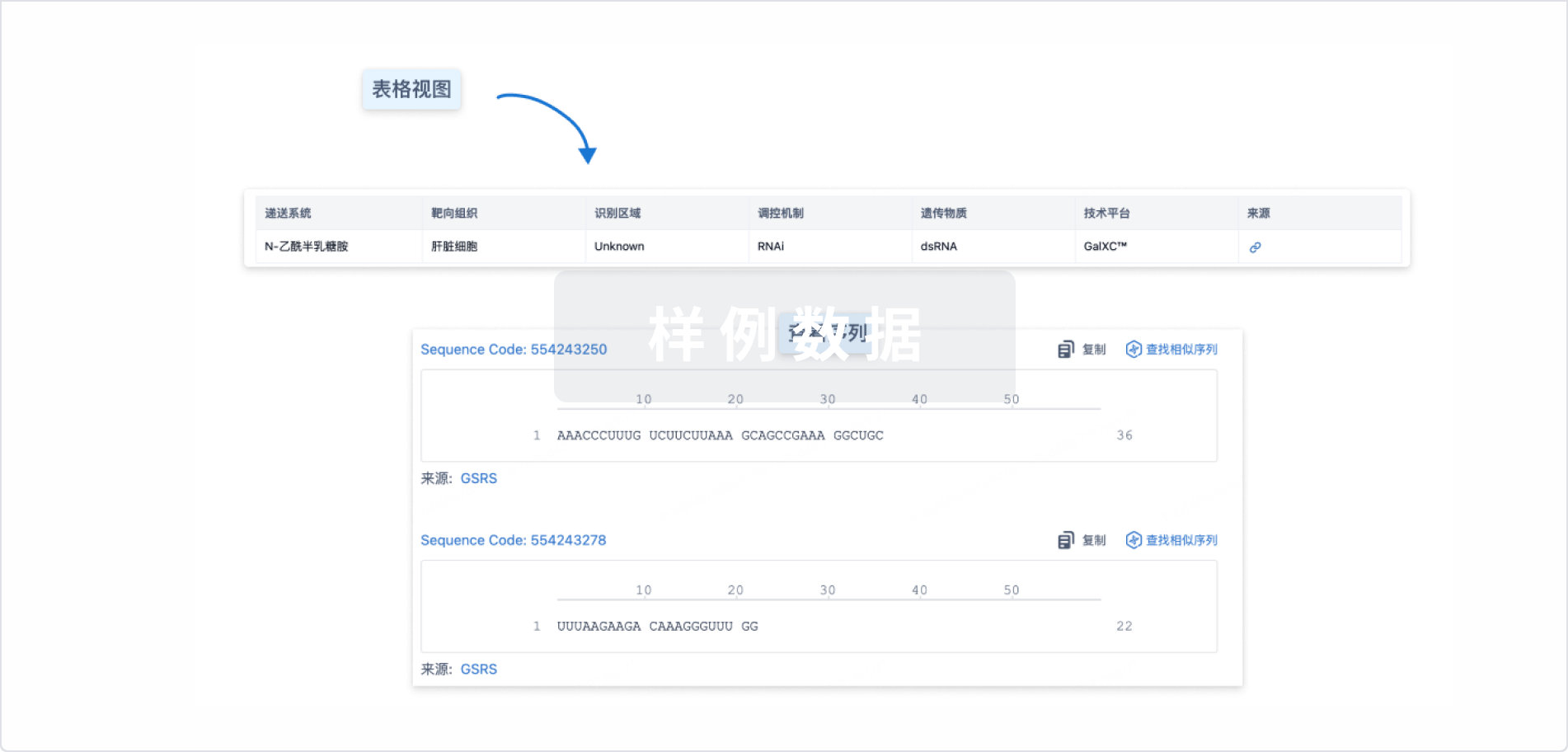
Sequence Code 538916969

来源: *****
关联
8
项与 Donidalorsen 相关的临床试验NCT05392114
An Open-Label, Long Term Safety and Efficacy Study of Donidalorsen in the Prophylactic Treatment of Hereditary Angioedema (HAE)
The purpose of this study is to evaluate the long-term safety and efficacy of donidalorsen in people with HAE and the effects of donidalorsen on the number of HAE attacks and their impact on quality of life (QoL).
开始日期2022-07-13 |
申办/合作机构 |
NCT05139810
A Phase 3 Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of ISIS 721744 in Patients With Hereditary Angioedema (HAE)
The purpose of this study was to evaluate the safety and efficacy of donidalorsen in participants with HAE and effect of donidalorsen on the quality and pattern of HAE attacks and their impact on quality of life (QoL).
开始日期2021-12-03 |
申办/合作机构 |
NCT04549922
Antisense Therapy to Block the Kallikrein-kinin Pathway in COVID-19: A Phase II Randomized Controlled Trial
Up to 1/3 of all patients infected with COVID-19 can develop complications that require hospitalization. Severe pneumonia associated with acute respiratory distress syndrome (ARDS) is the most threatening and feared complication of COVID-19 infection, with mortality rates close to 50% in some groups.
Autopsies between these severe cases reveal severe capillary involvement, with signs of intense inflammatory changes, microvascular thrombosis, endothelial injury and abnormal tissue repair. The available evidence suggests that abnormal activation or imbalance in the counter-regulation of the kallikrein-kinin system may play a central role in a positive feedback cycle, leading to consequent diffuse microangiopathy. Blockade of the kallikrein-kinin system can therefore prevent deterioration of lung function by reducing inflammation, edema and microthrombosis.
The objective of this phase IIb study is to assess the preliminary effects on the oxygenation parameters of an antisense oligonucleotide that inhibits pre-kallikrein synthesis in patients with moderate to severe COVID-19.
Autopsies between these severe cases reveal severe capillary involvement, with signs of intense inflammatory changes, microvascular thrombosis, endothelial injury and abnormal tissue repair. The available evidence suggests that abnormal activation or imbalance in the counter-regulation of the kallikrein-kinin system may play a central role in a positive feedback cycle, leading to consequent diffuse microangiopathy. Blockade of the kallikrein-kinin system can therefore prevent deterioration of lung function by reducing inflammation, edema and microthrombosis.
The objective of this phase IIb study is to assess the preliminary effects on the oxygenation parameters of an antisense oligonucleotide that inhibits pre-kallikrein synthesis in patients with moderate to severe COVID-19.
开始日期2020-10-19 |
申办/合作机构 |
100 项与 Donidalorsen 相关的临床结果
登录后查看更多信息
100 项与 Donidalorsen 相关的转化医学
登录后查看更多信息
100 项与 Donidalorsen 相关的专利(医药)
登录后查看更多信息
6
项与 Donidalorsen 相关的文献(医药)2025-08-01·ALLERGY
Patient‐Reported Outcomes in the Phase III OASIS‐HAE Study of Donidalorsen for Hereditary Angioedema
Article
作者: Treadwell, Sabrina ; Cohn, Danny M. ; Yarlas, Aaron ; Riedl, Marc A. ; Wang, Sophie ; Bordone, Laura ; Newman, Kenneth B.
ABSTRACT:
Background:
Hereditary angioedema (HAE) is a rare disease characterized by unpredictable, frequently severe swelling that negatively impacts patients' quality of life (QoL). In the phase III OASIS‐HAE study (NCT05139810), donidalorsen reduced HAE attack rate, increased disease control, and improved QoL. Here, we report further analysis of donidalorsen's impact on QoL and other patient‐reported outcomes (PROs).
Methods:
This double‐blind, placebo‐controlled study randomized patients with HAE to donidalorsen 80 mg or placebo once every 4 (Q4W) or 8 weeks (Q8W) over 24 weeks. PROs included Angioedema (AE)‐QoL Questionnaire, Angioedema Control Test (AECT), Patient Global Impression of Severity (PGIS), and Work Productivity and Activity Impairment Questionnaire plus Classroom Impairment Questions (WPAI+CIQ).
Results:
Ninety patients received donidalorsen Q4W (n = 45), donidalorsen Q8W (n = 23), or placebo (n = 22). A larger percentage of the donidalorsen Q4W group (88%) achieved clinically meaningful improvement (≥ 6‐point reduction) in AE‐QoL total score vs. placebo (45%). Both donidalorsen groups reported larger least‐squares mean (LSM) changes from baseline to week 24 vs. placebo in AE‐QoL functioning (difference: Q4W, −24.5; Q8W, −16.1), fears/shame (Q4W, −23.9; Q8W, −20.1), and nutrition (Q4W, −15.7; Q8W, −10.7) domains. Donidalorsen improved AECT scores vs. placebo (difference: Q4W, 6.0; Q8W, 4.1). A greater proportion of the donidalorsen Q4W group reported decreased disease severity vs. the placebo group (PGIS; 82% vs. 44%). Donidalorsen Q4W showed benefits vs. placebo in the presenteeism, overall work/school impairment, and activity impairment domains of the WPAI+CIQ.
Conclusions:
Donidalorsen significantly improved QoL and other PROs vs. placebo in patients with HAE.
2025-07-01·Journal of Allergy and Clinical Immunology-In Practice
Donidalorsen Treatment of Hereditary Angioedema in Patients Previously on Long-Term Prophylaxis
Article
作者: Lin, Tao ; Manning, Michael E ; Perego, Francesca ; Craig, Timothy ; Treadwell, Sabrina ; Lumry, William R ; Bordone, Laura ; Newman, Kenneth B ; Jacobs, Joshua S ; Yarlas, Aaron ; Riedl, Marc A ; Bernstein, Jonathan A ; Cohn, Danny M ; Banerji, Aleena ; Gierer, Selina ; Wedner, H James
BACKGROUND:
Hereditary angioedema (HAE) is a rare, potentially life-threatening disorder characterized by episodes of tissue swelling. Donidalorsen, an investigational ligand-conjugated antisense oligonucleotide, reduces plasma prekallikrein production.
OBJECTIVE:
We report an interim analysis on safety, efficacy, quality of life (QoL), and treatment preference and satisfaction from an ongoing open-label phase 3 study (OASISplus Switch cohort, NCT05392114).
METHODS:
Patients with HAE receiving stable doses (≥12 weeks) of lanadelumab, complement protein 1 inhibitor, or berotralstat switched to donidalorsen 80 mg subcutaneously every 4 weeks, using a predefined algorithm. The primary end point was the incidence and severity of treatment-emergent adverse events. Other end points included change in HAE attack rate, angioedema-QoL score, disease control (≥10 points on the Angioedema Control Test), and treatment preference and satisfaction at week 16, compared with baseline on prior treatment.
RESULTS:
A total of 65 patients were enrolled; 32 switched from lanadelumab, 22 from complement protein 1 inhibitor, and 11 from berotralstat. At cutoff, 58 were ongoing in the study (89%). Forty-five patients (70%) reported treatment-emergent adverse events; 62% were unrelated to donidalorsen. At week 16, total HAE attack rates had decreased by 62%. Hereditary angioedema attack rates decreased by 65%, 41%, and 73%, and mean angioedema-QoL scores improved by 8.4, 9.6, and 17.1 points for patients switching from lanadelumab, C1INH, and berotralstat, respectively. More patients reported well-controlled disease (93% vs 67%), and most patients preferred donidalorsen over their prior treatment, with improved treatment satisfaction.
CONCLUSIONS:
Donidalorsen was well tolerated, decreased HAE attack rate, and improved QoL and disease control. Most patients preferred donidalorsen over their prior treatment. Further analyses are planned at week 52.
2024-12-01·JOURNAL OF CRITICAL CARE
Antisense therapy to block the Kallikrein-kinin pathway in COVID-19: The ASKCOV randomized controlled trial
Article
作者: Buchele, Gustavo ; Gomes, Samara P C ; Santos, Maria Adelaide Dos ; Negrelli, Karina L ; Falavigna, Maicon ; Dal-Pizzol, Felipe ; de Freitas, Flávio Geraldo ; Miranda, Tamiris A ; Germano, Almir ; Gebara, Otávio ; Rosa, Regis G ; Santucci, Eliana Vieira ; Santos, Renato H N ; Zampieri, Fernando G ; Gomes, Jackeline O ; Valeis, Nanci ; Ishihara, Luciana M ; Azevedo, Luciano C P ; Cavalcanti, Alexandre B ; Berti, Isabele Ribeiro ; Machado, Flávia R ; Schettini, Daniel Almeida ; Westphal, Glauco Adrieno ; Janiszewski, Mariano ; Cohn, Danny M ; de Matos Soeiro, Alexandre ; Laranjeira, Ligia N ; Veiga, Viviane C ; de Souza Dantas, Vicente Cés ; Damiani, Lucas P
PURPOSE:
To assess the effect of antisense therapy to block kallikrein-kinin pathway in COVID-19 patients.
MATERIAL AND METHODS:
Randomized, placebo-controlled, double blind, controlled trial enrolling hospitalized COVID-19 patients that required supplementary oxygen to sustain peripheral oxygen saturation. Key exclusion criteria included use of mechanical ventilation or vasopressors, and patients with more than 10 days since symptom onset or more than 48 h of oxygen use. Patients were randomized to either one subcutaneous dose of ISIS721744, an antisense that blocks prekallikrein, or placebo. The primary outcome was the number of days alive and free of oxygen support up to 15 days (DAFOR15). Secondary endpoints included organ failure score, need and duration of mechanical ventilation up to 15 days, and all-cause mortality at 30 days. Exploratory endpoints included physiological parameters, biomarkers, and quality of life.
RESULTS:
From October 10, 2020, to December 09, 2020, 111 patients were randomized at thirteen sites in Brazil (56 to treatment and 55 to control group). Average age was 57.5 years, and most patients were male (68.5%). There were no significant differences in DAFOR15 between groups (5.9 ± 5.2 days for the intervention arm and 7.7 ± 5.1 for the control group; mean difference - 0.65, 95% confidence intervals from -2.95 to 1.36, p = 0.520).
CONCLUSION:
Antisense therapy designed to block the kallikrein-kinin pathway did not demonstrate clinical benefits in increasing days-alive without respiratory support at 15 days in patients with COVID-19 during the first wave in 2020.
CLINICALTRIALS:
GOV IDENTIFIER:
NCT04549922.
118
项与 Donidalorsen 相关的新闻(医药)2025-11-04
·医药观澜
编者按:寡核苷酸药物已成为全球新药研发的核心领域之一,近年来发展迅猛,在罕见病等多个领域得到快速应用。当前,全球超300款寡核苷酸疗法管线已进入临床开发阶段,有望在未来造福更多病患。为助力全球合作伙伴更高效地推动寡核苷酸药物从实验室走向临床,药明康德旗下WuXi TIDES平台围绕寡核苷酸、多肽及其相关化学偶联药物建立了一体化解决方案,覆盖定制合成、共价偶联、工艺开发和CMC等关键环节,赋能创新项目加速进入临床阶段。
寡核苷酸疗法持续在罕见病领域突破
寡核苷酸疗法正逐步成为罕见病治疗领域的重要突破方向。2025年第三季度,寡核苷酸疗法也持续迎来多项进展。
8月,美国FDA批准反义寡核苷酸配体偶联药物Dawnzera(donidalorsen),用于预防12岁及以上成人和儿童患者的遗传性血管性水肿(HAE)发作。据相关新闻稿,Dawnzera是FDA批准的首个用于HAE的RNA靶向药物。
9月,欧盟批准反义寡核苷酸(ASO)疗法Tryngolza(olezarsen)作为饮食控制的辅助疗法,用于治疗经遗传学确认的家族性乳糜微粒血症(FCS)成人患者。同期,该疗法用于治疗重度高甘油三酯血症(sHTG)的3期试验也迎来积极结果:每月一次的olezarsen使患者空腹甘油三酯较安慰剂平均下降72%,急性胰腺炎事件减少85%。这些进展表明,寡核苷酸疗法在罕见病治疗中的成功,为其向更广泛疾病领域的拓展奠定了基础。
此外,补体C5靶向siRNA疗法cemdisiran,在用以治疗成人全身性重症肌无力(gMG)的NIMBLE临床3期试验中达到主要终点。数据显示,每三个月皮下注射一次cemdisiran单药可平均抑制74%的补体活性,而与C5抗体pozelimab联用后,补体抑制率提升至近99%。同期,ASO疗法zilganersen也在亚历山大病(AxD)的关键试验中显著改善患者步行能力,成为首款在该疾病中显示出潜在疾病修饰作用的在研疗法。上述两款疗法预计将于2026年第一季度提交监管申请。
另一方面,用于治疗Dravet综合征的在研ASO疗法zorevunersen在为期三年的扩展研究中表现出持续的抗癫痫发作效果,并伴随认知与行为指标的持续改善,该疗法已在今年8月完成3期试验的首位患者给药。
在2025年三季度,FDA还向多款寡核苷酸疗法授予突破性疗法认定,适应症涵盖多种罕见疾病。其中包括适用于外显子44和51跳跃的杜氏肌营养不良症(DMD)的抗体-寡核苷酸偶联疗法del-zota与DYNE-251,以及用于天使综合征的ASO疗法apazunersen与ION582。
向更广泛适应症推进
寡核苷酸疗法的应用领域正从罕见病向常见疾病拓展。7月,FDA批准诺华(Novartis)的siRNA疗法Leqvio(inclisiran)扩展适应症,作为单药与饮食控制和运动联合使用,以降低成人高胆固醇血症患者的低密度脂蛋白胆固醇(LDL-C)水平。该药物每年仅需给药两次。此次标签更新是FDA基于该PCSK9靶向疗法降低LDL-C的积极数据主动发起。
在代谢性疾病领域,用于治疗代谢功能障碍相关脂肪性肝炎(MASH)的ASO疗法ION224也在三季度获得积极的2期结果:最高剂量组中有近60%的患者实现MASH疾病活动度改善,安慰剂组仅为19%。
同期,两款分别针对肥胖与阿尔茨海默病的siRNA疗法RN3161与ARO-MAPT于9月在澳大利亚和新西兰提交临床试验申请,标志着寡核苷酸疗法正向更广泛疾病领域推进。
在癌症领域也有多项进展。PD-1靶向siRNA疗法PH-762在皮肤癌1b期试验中,13例皮肤鳞状细胞癌(cSCC)患者中有6人在接受治疗后达到病理学完全缓解或接近完全缓解。另外,DNA癌症疫苗SCIB1与其改良版iSCIB1+在晚期不可切除黑色素瘤患者中展现亮眼疗效。临床2期试验结果显示,当两者与当前标准免疫检查点抑制剂联用时,疾病控制率(DCR)高达88.0%。
9月,两款分别针对肥胖与阿尔茨海默病的siRNA疗法RN3161与ARO-MAPT,也分别在澳洲与新西兰递交临床试验申请,意味着着寡核苷酸疗法正向更广泛适应症领域推进。
高额合作加码,彰显产业信心
三季度以来,寡核苷酸领域的商业研发合作也有诸多进展,多家企业通过高额合作加码siRNA领域布局。
9月,诺华接连达成两项大额siRNA疗法授权合作:其一,与Arrowhead Pharmaceuticals就后者开发的α-突触核蛋白靶向siRNA疗法ARO-SNCA达成总额高达20亿美元的合作。该疗法处于临床前阶段,拟开发治疗包括帕金森病在内的突触核蛋白病。其二,与Argo Biopharma就多项心血管siRNA管线达成合作,合作总额最高可达52亿美元,进一步夯实其在心血管代谢管线。
同时,Arrowhead Pharmaceuticals控股子公司维亚臻(Visirna Therapeutics)与赛诺菲达成最高2.65亿美元的合作:赛诺菲将获得siRNA疗法plozasiran(VSA001)在大中华区的开发与商业化独家权利。该疗法通过抑制载脂蛋白C-III(APOC3)的产生,用于治疗家族性乳糜微粒血症综合征及重度高甘油三酯血症。
此外,专注于开发RNA疗法的Arnatar Therapeutics宣布已于2024年完成5200万美元A轮融资。该公司专有的DARGER平台将siRNA沉默技术与基于ASO的基因上调技术相结合,开发具双重作用机制的RNA疗法,针对心脏代谢、肝、肾及中枢神经系统等疾病。
总而言之,2025年第三季度,寡核苷酸疗法在多个疾病领域持续取得突破,展现出从罕见病向更广泛疾病领域拓展的明确趋势。同时,产业涌现出的高额融资、合作等趋势,显示产业对这一领域的信心。
作为医药创新的赋能者,药明康德化学业务旗下专注于寡核苷酸和多肽及相关化学偶联药物的新分子业务平台——WuXi TIDES平台,围绕siRNA、ASO等寡核苷酸疗法,建立了化合物合成、工艺开发及生产的一站式服务平台,覆盖从药物发现、CMC开发,到商业化生产的全生命周期,加速将合作伙伴的创新构想转化为现实,更好地造福全球病患。以下案例将展示WuXi TIDES的一体化平台如何加速合作伙伴ASO药物的开发进程。
2023年,一家生物技术公司与WuXi TIDES合作进行ASO药物的早期筛选研究,WuXi TIDES的药物化学团队为其提供了超过400种携带骨架化学修饰的ASO化合物,以协助确定最具前景的分子。然而,早期研究发现,创新骨架修饰导致候选化合物中出现新的杂质。在最初的合成过程中,这些杂质占比高达25%,不仅降低了产率和纯化效率,还可能带来潜在毒性,给后续临床开发带来挑战。
面对这一难题,WuXi TIDES药物化学团队和工艺研发团队密切配合,从两个方向入手解决问题。一方面,药物化学团队与合作伙伴共同探索杂质产生的潜在原因,设计出定制化的amidite和分子砌块,规避杂质产生的关键合成机制,并快速生产这些新分子砌块,协助工艺研发团队加速验证工艺设计策略,以有效地控制杂质。此外,工艺研发团队通过优化工艺参数,系统性地降低了杂质的产生。最终,经过持续工艺优化,杂质占比成功从25%降低至5%,同时最终收率也从最初的0.5 g/mol提高到3.4 g/mol。
在该项目中,WuXi TIDES各团队高效协作,不仅在12个月内完成了先导化合物的优化、工艺开发及GMP生产,更帮助合作伙伴基于数据进行快速决策,选出综合效力、稳定性和开发潜力俱佳的ASO候选化合物,为后续临床研究奠定了坚实基础。随着越来越多的ASO以及其他寡核苷酸药物进入临床开发,这种产业协同模式将成为加快研发步伐的重要推动力。
免责声明:本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。
版权说明:欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「医药观澜」微信公众号回复“转载”,获取转载须知。
寡核苷酸临床3期siRNA信使RNA
2025-11-03
·医药观澜
编者按:寡核苷酸药物已成为全球新药研发的核心领域之一,近年来发展迅猛,在罕见病等多个领域得到快速应用。当前,全球超300款寡核苷酸疗法管线已进入临床开发阶段,有望在未来造福更多病患。为助力全球合作伙伴更高效地推动寡核苷酸药物从实验室走向临床,药明康德旗下WuXi TIDES平台围绕寡核苷酸、多肽及其相关化学偶联药物建立了一体化解决方案,覆盖定制合成、共价偶联、工艺开发和CMC等关键环节,赋能创新项目加速进入临床阶段。
寡核苷酸疗法持续在罕见病领域突破
寡核苷酸疗法正逐步成为罕见病治疗领域的重要突破方向。2025年第三季度,寡核苷酸疗法也持续迎来多项进展。
8月,美国FDA批准反义寡核苷酸配体偶联药物Dawnzera(donidalorsen),用于预防12岁及以上成人和儿童患者的遗传性血管性水肿(HAE)发作。据相关新闻稿,Dawnzera是FDA批准的首个用于HAE的RNA靶向药物。
9月,欧盟批准反义寡核苷酸(ASO)疗法Tryngolza(olezarsen)作为饮食控制的辅助疗法,用于治疗经遗传学确认的家族性乳糜微粒血症(FCS)成人患者。同期,该疗法用于治疗重度高甘油三酯血症(sHTG)的3期试验也迎来积极结果:每月一次的olezarsen使患者空腹甘油三酯较安慰剂平均下降72%,急性胰腺炎事件减少85%。这些进展表明,寡核苷酸疗法在罕见病治疗中的成功,为其向更广泛疾病领域的拓展奠定了基础。
此外,补体C5靶向siRNA疗法cemdisiran,在用以治疗成人全身性重症肌无力(gMG)的NIMBLE临床3期试验中达到主要终点。数据显示,每三个月皮下注射一次cemdisiran单药可平均抑制74%的补体活性,而与C5抗体pozelimab联用后,补体抑制率提升至近99%。同期,ASO疗法zilganersen也在亚历山大病(AxD)的关键试验中显著改善患者步行能力,成为首款在该疾病中显示出潜在疾病修饰作用的在研疗法。上述两款疗法预计将于2026年第一季度提交监管申请。
另一方面,用于治疗Dravet综合征的在研ASO疗法zorevunersen在为期三年的扩展研究中表现出持续的抗癫痫发作效果,并伴随认知与行为指标的持续改善,该疗法已在今年8月完成3期试验的首位患者给药。
在2025年三季度,FDA还向多款寡核苷酸疗法授予突破性疗法认定,适应症涵盖多种罕见疾病。其中包括适用于外显子44和51跳跃的杜氏肌营养不良症(DMD)的抗体-寡核苷酸偶联疗法del-zota与DYNE-251,以及用于天使综合征的ASO疗法apazunersen与ION582。
向更广泛适应症推进
寡核苷酸疗法的应用领域正从罕见病向常见疾病拓展。7月,FDA批准诺华(Novartis)的siRNA疗法Leqvio(inclisiran)扩展适应症,作为单药与饮食控制和运动联合使用,以降低成人高胆固醇血症患者的低密度脂蛋白胆固醇(LDL-C)水平。该药物每年仅需给药两次。此次标签更新是FDA基于该PCSK9靶向疗法降低LDL-C的积极数据主动发起。
在代谢性疾病领域,用于治疗代谢功能障碍相关脂肪性肝炎(MASH)的ASO疗法ION224也在三季度获得积极的2期结果:最高剂量组中有近60%的患者实现MASH疾病活动度改善,安慰剂组仅为19%。
同期,两款分别针对肥胖与阿尔茨海默病的siRNA疗法RN3161与ARO-MAPT于9月在澳大利亚和新西兰提交临床试验申请,标志着寡核苷酸疗法正向更广泛疾病领域推进。
在癌症领域也有多项进展。PD-1靶向siRNA疗法PH-762在皮肤癌1b期试验中,13例皮肤鳞状细胞癌(cSCC)患者中有6人在接受治疗后达到病理学完全缓解或接近完全缓解。另外,DNA癌症疫苗SCIB1与其改良版iSCIB1+在晚期不可切除黑色素瘤患者中展现亮眼疗效。临床2期试验结果显示,当两者与当前标准免疫检查点抑制剂联用时,疾病控制率(DCR)高达88.0%。
9月,两款分别针对肥胖与阿尔茨海默病的siRNA疗法RN3161与ARO-MAPT,也分别在澳洲与新西兰递交临床试验申请,意味着着寡核苷酸疗法正向更广泛适应症领域推进。
高额合作加码,彰显产业信心
三季度以来,寡核苷酸领域的商业研发合作也有诸多进展,多家企业通过高额合作加码siRNA领域布局。
9月,诺华接连达成两项大额siRNA疗法授权合作:其一,与Arrowhead Pharmaceuticals就后者开发的α-突触核蛋白靶向siRNA疗法ARO-SNCA达成总额高达20亿美元的合作。该疗法处于临床前阶段,拟开发治疗包括帕金森病在内的突触核蛋白病。其二,与Argo Biopharma就多项心血管siRNA管线达成合作,合作总额最高可达52亿美元,进一步夯实其在心血管代谢管线。
同时,Arrowhead Pharmaceuticals控股子公司维亚臻(Visirna Therapeutics)与赛诺菲达成最高2.65亿美元的合作:赛诺菲将获得siRNA疗法plozasiran(VSA001)在大中华区的开发与商业化独家权利。该疗法通过抑制载脂蛋白C-III(APOC3)的产生,用于治疗家族性乳糜微粒血症综合征及重度高甘油三酯血症。
此外,专注于开发RNA疗法的Arnatar Therapeutics宣布已于2024年完成5200万美元A轮融资。该公司专有的DARGER平台将siRNA沉默技术与基于ASO的基因上调技术相结合,开发具双重作用机制的RNA疗法,针对心脏代谢、肝、肾及中枢神经系统等疾病。
总而言之,2025年第三季度,寡核苷酸疗法在多个疾病领域持续取得突破,展现出从罕见病向更广泛疾病领域拓展的明确趋势。同时,产业涌现出的高额融资、合作等趋势,显示产业对这一领域的信心。
作为医药创新的赋能者,药明康德化学业务旗下专注于寡核苷酸和多肽及相关化学偶联药物的新分子业务平台——WuXi TIDES平台,围绕siRNA、ASO等寡核苷酸疗法,建立了化合物合成、工艺开发及生产的一站式服务平台,覆盖从药物发现、CMC开发,到商业化生产的全生命周期,加速将合作伙伴的创新构想转化为现实,更好地造福全球病患。以下案例将展示WuXi TIDES的一体化平台如何加速合作伙伴ASO药物的开发进程。
2023年,一家生物技术公司与WuXi TIDES合作进行ASO药物的早期筛选研究,WuXi TIDES的药物化学团队为其提供了超过400种携带骨架化学修饰的ASO化合物,以协助确定最具前景的分子。然而,早期研究发现,创新骨架修饰导致候选化合物中出现新的杂质。在最初的合成过程中,这些杂质占比高达25%,不仅降低了产率和纯化效率,还可能带来潜在毒性,给后续临床开发带来挑战。
面对这一难题,WuXi TIDES药物化学团队和工艺研发团队密切配合,从两个方向入手解决问题。一方面,药物化学团队与合作伙伴共同探索杂质产生的潜在原因,设计出定制化的amidite和分子砌块,规避杂质产生的关键合成机制,并快速生产这些新分子砌块,协助工艺研发团队加速验证工艺设计策略,以有效地控制杂质。此外,工艺研发团队通过优化工艺参数,系统性地降低了杂质的产生。最终,经过持续工艺优化,杂质占比成功从25%降低至5%,同时最终收率也从最初的0.5 g/mol提高到3.4 g/mol。
在该项目中,WuXi TIDES各团队高效协作,不仅在12个月内完成了先导化合物的优化、工艺开发及GMP生产,更帮助合作伙伴基于数据进行快速决策,选出综合效力、稳定性和开发潜力俱佳的ASO候选化合物,为后续临床研究奠定了坚实基础。随着越来越多的ASO以及其他寡核苷酸药物进入临床开发,这种产业协同模式将成为加快研发步伐的重要推动力。
免责声明:本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。
版权说明:欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「医药观澜」微信公众号回复“转载”,获取转载须知。
寡核苷酸临床3期siRNA上市批准信使RNA
2025-10-29
- TRYNGOLZA® generated $32 million in net product sales in the third quarter 2025 -
- DAWNZERA™ (donidalorsen) launch off to encouraging start -
- Olezarsen significantly reduced triglycerides and acute pancreatitis events in severe hypertriglyceridemia (sHTG) in landmark Phase 3 studies; sNDA submission on track by year-end –
- Positive pivotal zilganersen results in Alexander disease position Ionis for first independent neurology launch in 2026 -
- Increasing 2025 financial guidance driven by continued strength across the business –
CARLSBAD, Calif. --(BUSINESS WIRE)--Oct. 29, 2025-- Ionis Pharmaceuticals, Inc. (Nasdaq: IONS) (the “Company”) today reported financial results and provided key updates for the third quarter ended September 30, 2025 .
"The third quarter was a watershed moment for Ionis, as we made important progress advancing our Ionis-owned medicines. With two independent launches now underway, and two more anticipated in 2026, we are delivering on our goal to bring a steady cadence of new medicines to people in need,” said Brett P. Monia , Ph.D., chief executive officer of Ionis. “Last month, we announced groundbreaking, positive topline Phase 3 results for olezarsen in severe hypertriglyceridemia and for zilganersen in Alexander disease, with regulatory filings planned in the coming months. Our approved and late-stage portfolio continues to deliver — positioning Ionis for substantial growth while, most importantly, offering the opportunity to profoundly improve the lives of people with serious diseases."
Third Quarter 2025 Summary Financial Results(1):
Three months ended
Nine months ended
September 30 ,
September 30 ,
2025
2024
2025
2024
(amounts in millions)
Total revenue
$
157
$
134
$
740
$
479
Operating expenses
$
317
$
282
$
907
$
843
Operating expenses on a non-GAAP basis
$
286
$
250
$
816
$
749
Loss from operations
($
160
)
($
148
)
($
167
)
($
364
)
Loss from operations on a non-GAAP basis
($
129
)
($
116
)
($
76
)
($
270
)
(1)
Reconciliation of GAAP to non-GAAP basis contained later in this release.
Recent Financial Highlights
Revenue increased 17% in the third quarter of 2025 and increased 55% in the nine months ended September 30, 2025 , compared to the same periods last year, driven by the continued successful launch of TRYNGOLZA and increased royalty revenues. Contributing to the year-to-date increase, Ionis earned a $280 million upfront payment for the global license of sapablursen to Ono Pharmaceutical Co., Ltd. in the second quarter of 2025 Operating expenses on a non-GAAP basis increased 14% in the third quarter of 2025 and increased 9% in the nine months ended September 30, 2025 , compared to the same periods last year, primarily due to investments related to commercialization efforts for TRYNGOLZA, DAWNZERA and WAINUA Increasing 2025 financial guidance reflects strong overall revenue performance experienced year-to-date and fourth quarter outlook, including strong momentum seen with TRYNGOLZA revenues:
Full Year 2025 Guidance
Previous Guidance
New Guidance
Total Revenue
$825-850 million
$875-900 million
TRYNGOLZA product sales, net
$75-80 million
$85-95 million
Operating loss on a non-GAAP basis
$300-325 million
$275-300 million
Cash, cash equivalents and short-term investments
~$2.0 billion
> $2.1 billion
Third Quarter 2025 Financial Results
"In the third quarter of 2025, we delivered strong revenue performance, highlighted by TRYNGOLZA’s nearly 70% increase over the prior quarter. As a result of this strength and our fourth quarter outlook, we are increasing our financial guidance again for 2025," said Elizabeth L. Hougen , chief financial officer of Ionis. "Looking ahead, we expect the 2026 independent launches of olezarsen in severe hypertriglyceridemia and zilganersen in Alexander disease to further strengthen our commercial portfolio. We anticipate that growth in our product revenues coupled with additional partner revenues will position Ionis to achieve cash flow breakeven in 2028 and generate substantial and sustainable positive cash flow for years to come."
Recent Highlights - Wholly Owned Medicines
TRYNGOLZA® (olezarsen), the first and only FDA approved treatment for adults living with familial chylomicronemia syndrome (FCS) as an adjunct to diet Generated net product sales of $32 million in the third quarter of 2025, its third full quarter on the market, a nearly 70% increase over the prior quarter, and $57 million in the nine months ended September 30, 2025 Approved in the European Union (EU) as an adjunct to diet in adult patients for the treatment of genetically confirmed FCS ; Sobi anticipates launching in the fourth quarter 2025 Olezarsen demonstrated positive topline results in the pivotal Phase 3 CORE and CORE2 studies in sHTG Olezarsen demonstrated a highly statistically significant placebo-adjusted mean reduction in fasting triglycerides of up to 72% and a highly statistically significant reduction in acute pancreatitis events of 85% with favorable safety and tolerability sNDA submission on track for the end of 2025 with approval anticipated in the fourth quarter of 2026 Detailed data to be presented at the American Heart Association Conference on November 8, 2025 , in a late-breaking session DAWNZERA™ (donidalorsen) was approved on August 21, 2025 , by the FDA for prophylaxis to prevent attacks of hereditary angioedema ( HAE) in adult and pediatric patients 12 years of age and older First and only RNA-targeted prophylactic therapy that has the potential to offer durable efficacy, a favorable safety and tolerability profile, and the longest available dosing interval, with self-administration via autoinjector every four or eight weeks U.S. launch underway and off to an encouraging start Currently under regulatory review in the EU Zilganersen demonstrated positive results in the pivotal study in children and adults with Alexander disease (AxD), a rare, progressive, and often fatal neurological disorder with no approved disease-modifying treatments Zilganersen 50 mg demonstrated statistically significant and clinically meaningful stabilization on the primary endpoint of gait speed as assessed by the 10-Meter Walk Test (10MWT), compared to control at week 61 (mean difference 33.3%) with favorable safety and tolerability Additional data from the pivotal study in children and adults living with Alexander disease were presented at the Child Neurology Society Annual Meeting in October 2025 NDA submission planned for the first quarter of 2026 with approval anticipated next year ION582 granted Breakthrough Therapy designation from FDA for the treatment of Angelman syndrome Phase 3 REVEAL study expected to be fully enrolled in 2026 Phase 2 HALOS study showed continued improvement across multiple functional measures versus natural history through 18 months including expressive communication
Generated net product sales of $32 million in the third quarter of 2025, its third full quarter on the market, a nearly 70% increase over the prior quarter, and $57 million in the nine months ended September 30, 2025 Approved in the European Union (EU) as an adjunct to diet in adult patients for the treatment of genetically confirmed FCS ; Sobi anticipates launching in the fourth quarter 2025
Olezarsen demonstrated a highly statistically significant placebo-adjusted mean reduction in fasting triglycerides of up to 72% and a highly statistically significant reduction in acute pancreatitis events of 85% with favorable safety and tolerability sNDA submission on track for the end of 2025 with approval anticipated in the fourth quarter of 2026 Detailed data to be presented at the American Heart Association Conference on November 8, 2025 , in a late-breaking session
First and only RNA-targeted prophylactic therapy that has the potential to offer durable efficacy, a favorable safety and tolerability profile, and the longest available dosing interval, with self-administration via autoinjector every four or eight weeks U.S. launch underway and off to an encouraging start Currently under regulatory review in the EU
Zilganersen 50 mg demonstrated statistically significant and clinically meaningful stabilization on the primary endpoint of gait speed as assessed by the 10-Meter Walk Test (10MWT), compared to control at week 61 (mean difference 33.3%) with favorable safety and tolerability Additional data from the pivotal study in children and adults living with Alexander disease were presented at the Child Neurology Society Annual Meeting in October 2025 NDA submission planned for the first quarter of 2026 with approval anticipated next year
Phase 3 REVEAL study expected to be fully enrolled in 2026 Phase 2 HALOS study showed continued improvement across multiple functional measures versus natural history through 18 months including expressive communication
Recent Highlights – Partnered Medicines
WAINUA® (eplontersen) (WAINZUA in EU) for the treatment of adults with polyneuropathy of hereditary transthyretin-mediated amyloidosis (ATTRv-PN) generated sales of $59 million and $143 million resulting in royalty revenue of $13 million and $33 million in the third quarter and the nine months ended September 30, 2025 , respectively Launches underway in numerous regions, including the EU; additional submissions in progress to expand WAINUA access globally SPINRAZA® (nusinersen) for the treatment of spinal muscular atrophy (SMA) generated global sales of $374 million and $1.2 billion resulting in royalty revenue of $56 million and $158 million in the third quarter and the nine months ended September 30, 2025 , respectively
Launches underway in numerous regions, including the EU; additional submissions in progress to expand WAINUA access globally
Revenue
Ionis’ revenue was comprised of the following:
Three months ended
Nine months ended
September 30 ,
September 30 ,
2025
2024
2025
2024
Revenue:
(amounts in millions)
Commercial revenue:
Product sales, net:
TRYNGOLZA sales, net
$
32
$
-
$
57
$
-
Total product sales, net
32
-
57
-
Royalty revenue:
SPINRAZA royalties
56
57
158
152
WAINUA royalties
13
5
33
10
Other royalties
7
5
19
18
Total royalty revenue
76
67
210
180
Other commercial revenue
8
9
27
27
Total commercial revenue
116
76
294
207
Research and development revenue:
Collaborative agreement revenue
31
45
414
237
WAINUA joint development revenue
10
13
32
35
Total research and development revenue
41
58
446
272
Total revenue
$
157
$
134
$
740
$
479
Commercial revenue for the third quarter and the nine months ended September 30, 2025 , increased 53% and 42%, respectively, compared to the same periods in 2024. This increase was primarily driven by TRYNGOLZA product sales. Higher royalty revenue also contributed to the year over year increase.
The remainder of the Company’s revenue came from programs under its R&D collaborations, including a $280 million upfront payment for the global license of sapablursen to Ono Pharmaceutical Co., Ltd. in the second quarter of 2025, reflecting the value that Ionis’ pipeline and technology continues to generate.
Operating Expenses
SG&A expenses increased as anticipated for the third quarter and the nine months ended September 30, 2025 , compared to the same periods in 2024, primarily due to the launches of TRYNGOLZA, DAWNZERA and WAINUA. This increase was partially offset by a decrease in R&D expenses as several late-stage studies ended. Overall, this led to a modest year-over-year increase in total operating expenses, which was in line with expectations.
Balance Sheet
As of September 30, 2025 , Ionis’ cash, cash equivalents and short-term investments were $2.2 billion , compared to $2.3 billion on December 31, 2024 . Ionis’ working capital decreased over the same period primarily due to the reclassification of the Company’s 0% convertible notes as a current liability.
Webcast and Other Updates
Management will host a conference call and webcast to discuss Ionis’ third quarter 2025 results at 11:30 a.m. Eastern time on Wednesday, October 29, 2025 . Interested parties may access the webcast here. A webcast replay will be available for a limited time at the same address. To access the Company’s third quarter 2025 earnings slides click here.
Ionis’ Marketed Medicines
INDICATION for TRYNGOLZA® (olezarsen)
TRYNGOLZA® (olezarsen) was approved by the U.S. Food and Drug Administration as an adjunct to diet to reduce triglycerides in adults with familial chylomicronemia syndrome (FCS).
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
TRYNGOLZA is contraindicated in patients with a history of serious hypersensitivity to TRYNGOLZA or any of the excipients in TRYNGOLZA. Hypersensitivity reactions requiring medical treatment have occurred.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions (including symptoms of bronchospasm, diffuse erythema, facial swelling, urticaria, chills and myalgias) have been reported in patients treated with TRYNGOLZA. Advise patients on the signs and symptoms of hypersensitivity reactions and instruct patients to promptly seek medical attention and discontinue use of TRYNGOLZA if hypersensitivity reactions occur.
ADVERSE REACTIONS
The most common adverse reactions (incidence >5% of TRYNGOLZA-treated patients and >3% higher frequency than placebo) were injection site reactions, decreased platelet count and arthralgia.
Please see full Prescribing Information for TRYNGOLZA.
INDICATION for DAWNZERATM (donidalorsen)
DAWNZERA™ (donidalorsen) was approved by the U.S. Food and Drug Administration for prophylaxis to prevent attacks of hereditary angioedema ( HAE) in adult and pediatric patients 12 years of age and older.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
DAWNZERA is contraindicated in patients with a history of serious hypersensitivity reactions, including anaphylaxis, to donidalorsen or any of the excipients in DAWNZERA.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, have been reported in patients treated with DAWNZERA. If signs and symptoms of serious hypersensitivity reactions occur, discontinue DAWNZERA and institute appropriate therapy.
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5%) are injection site reactions, upper respiratory tract infection, urinary tract infection, and abdominal discomfort.
Please see full Prescribing Information for DAWNZERA.
INDICATION for WAINUA® (eplontersen)
WAINUA injection, for subcutaneous use, 45 mg is indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults.
IMPORTANT SAFETY INFORMATION for WAINUA® (eplontersen)
WARNINGS AND PRECAUTIONS
Reduced Serum Vitamin A Levels and Recommended Supplementation WAINUA leads to a decrease in serum vitamin A levels. Supplement with recommended daily allowance of vitamin A. Refer patient to an ophthalmologist if ocular symptoms suggestive of vitamin A deficiency occur.
ADVERSE REACTIONS
Most common adverse reactions (≥9% in WAINUA-treated patients) were vitamin A decreased (15%) and vomiting (9%).
Please see link to U.S. Full Prescribing Information for WAINUA.
For more information about SPINRAZA and QALSODY, visit https://www.spinraza.com/ and https://www.qalsody.com/, respectively. QALSODY is approved under accelerated approval based on reduction in plasma neurofilament light chain (NfL) observed in patients treated with QALSODY. Continued approval may be contingent upon verification of clinical benefit in confirmatory trial(s).
About Ionis Pharmaceuticals, Inc.
For three decades, Ionis has invented medicines that bring better futures to people with serious diseases. Ionis currently has marketed medicines and a leading pipeline in neurology, cardiometabolic disease and select areas of high patient need. As the pioneer in RNA-targeted medicines, Ionis continues to drive innovation in RNA therapies in addition to advancing new approaches in gene editing. A deep understanding of disease biology and industry-leading technology propels our work, coupled with a passion and urgency to deliver life-changing advances for patients. To learn more about Ionis, visit Ionis.com and follow us on X (Twitter), LinkedIn and Instagram.
Ionis’ Forward-looking Statement
This press release includes forward-looking statements regarding Ionis’ business, financial guidance and the therapeutic and commercial potential of our commercial medicines, additional medicines in development, technologies and our expectations regarding development and regulatory milestones. Any statement describing Ionis’ goals, expectations, financial or other projections, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties including those inherent in the process of discovering, developing and commercializing medicines that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such medicines. Ionis’ forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ materially from those expressed or implied by such forward-looking statements. Although Ionis’ forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by Ionis. Except as required by law, we undertake no obligation to update any forward-looking statements for any reason. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Ionis' programs are described in additional detail in Ionis' annual report on Form 10-K for the year ended December 31, 2024 , and most recent Form 10-Q, which are on file with the Securities and Exchange Commission. Copies of these and other documents are available from the Company.
In this press release, unless the context requires otherwise, “Ionis,” “Company,” “we,” “our” and “us” all refer to Ionis Pharmaceuticals and its subsidiaries.
IONIS® is a registered trademark of Ionis Pharmaceuticals, Inc. TRYNGOLZA® is a registered trademark of Ionis Pharmaceuticals, Inc. DAWNZERATM is a trademark of Ionis Pharmaceuticals, Inc. AKCEATM is a trademark of Akcea Therapeutics, Inc. TEGSEDITM is a trademark of Akcea Therapeutics, Inc. WAYLIVRATM is a trademark of Akcea Therapeutics, Inc. SPINRAZA® and QALSODY® are registered trademarks of Biogen. WAINUA® is a registered trademark of the AstraZeneca group of companies.
IONIS PHARMACEUTICALS, INC.
SELECTED FINANCIAL INFORMATION
Condensed Consolidated Statements of Operations
(In Millions, Except Per Share Data)
Three months ended
Nine months ended
September 30 ,
September 30 ,
2025
2024
2025
2024
(unaudited)
Revenue:
Commercial revenue:
Product sales, net
$
32
$
-
$
57
$
-
Royalty revenue
76
67
210
180
Other commercial revenue
8
9
27
27
Total commercial revenue
116
76
294
207
Research and development revenue:
Collaborative agreement revenue
31
45
414
237
WAINUA joint development revenue
10
13
32
35
Total research and development revenue
41
58
446
272
Total revenue
157
134
740
479
Expenses:
Cost of sales
2
1
8
7
Research, development and patent
218
220
636
656
Selling, general and administrative
97
61
263
180
Total operating expenses
317
282
907
843
Loss from operations
(160
)
(148
)
(167
)
(364
)
Other income (expense):
Interest expense related to the sale of future royalties
(18
)
(19
)
(55
)
(55
)
Other income, net
49
23
70
66
Loss before income tax benefit
(129
)
(144
)
(152
)
(353
)
Income tax benefit
-
4
-
3
Net loss
($
129
)
($
140
)
($
152
)
($
350
)
Basic and diluted net loss per share
($
0.80
)
($
0.95
)
($
0.95
)
($
2.38
)
Shares used in computing basic and diluted net loss per share
160
149
159
147
IONIS PHARMACEUTICALS, INC.
Reconciliation of GAAP to Non-GAAP Basis:
Condensed Consolidated Operating Expenses, Loss From Operations, and Net Loss
(In Millions)
Three months ended September 30 ,
Nine months ended September 30 ,
2025
2024
2025
2024
(unaudited)
As reported research, development and patent expenses according to GAAP
$
218
$
220
$
636
$
656
Excluding compensation expense related to equity awards
(21
)
(22
)
(61
)
(67
)
Non-GAAP research, development and patent expenses
$
197
$
198
$
575
$
589
As reported selling, general and administrative expenses according to GAAP
$
97
$
61
$
263
$
180
Excluding compensation expense related to equity awards
(10
)
(10
)
(29
)
(26
)
Non-GAAP selling, general and administrative expenses
$
87
$
51
$
234
$
154
As reported operating expenses according to GAAP
$
317
$
282
$
907
$
843
Excluding compensation expense related to equity awards
(31
)
(32
)
(91
)
(94
)
Non-GAAP operating expenses
$
286
$
250
$
816
$
749
As reported loss from operations according to GAAP
($
160
)
($
148
)
($
167
)
($
364
)
Excluding compensation expense related to equity awards
(31
)
(32
)
(91
)
(94
)
Non-GAAP loss from operations
($
129
)
($
116
)
($
76
)
($
270
)
As reported net loss according to GAAP
($
129
)
($
140
)
($
152
)
($
350
)
Excluding compensation expense related to equity awards and related tax effects
(31
)
(32
)
(91
)
(94
)
Non-GAAP net loss
($
98
)
($
108
)
($
61
)
($
256
)
Reconciliation of GAAP to Non-GAAP Basis
As illustrated in the Selected Financial Information in this press release, non-GAAP operating expenses, non-GAAP loss from operations, and non-GAAP net loss were adjusted from GAAP to exclude compensation expense related to equity awards and the related tax effects. Compensation expense related to equity awards are non-cash. These measures are provided as supplementary information and are not a substitute for financial measures calculated in accordance with GAAP. Ionis reports these non-GAAP results to better enable financial statement users to assess and compare its historical performance and project its future operating results and cash flows. Further, the presentation of Ionis’ non-GAAP results is consistent with how Ionis’ management internally evaluates the performance of its operations.
IONIS PHARMACEUTICALS, INC.
Condensed Consolidated Balance Sheets
(In Millions)
September 30 ,
December 31 ,
2025
2024
(unaudited)
Assets:
Cash, cash equivalents and short-term investments
$
2,240
$
2,298
Contracts receivable
25
92
Other current assets
254
230
Property, plant and equipment, net
106
94
Right-of-use assets
242
162
Other assets
166
127
Total assets
$
3,033
$
3,003
Liabilities and stockholders’ equity:
Current portion of deferred contract revenue
$
77
$
79
0% convertible senior notes, net – current
631
-
Other current liabilities
195
229
1.75% convertible senior notes, net
567
565
0% convertible senior notes, net
-
629
Liability related to sale of future royalties, net
545
542
Long-term lease liabilities
263
162
Long-term obligations, less current portion
29
52
Long-term deferred contract revenue
108
157
Total stockholders’ equity
618
588
Total liabilities and stockholders’ equity
$
3,033
$
3,003
Key 2025 and 2026 Value Driving Events(1)
New Product Launches
Program
Indication
2025
2026
DAWNZERA ( U.S. )
HAE
Achieved
TRYNGOLZA ( U.S. )
FCS
Achieved
WAINZUA (EU)
ATTRv-PN
Achieved
Olezarsen ( U.S. )
sHTG
•
Zilganersen ( U.S. )
Alexander disease
•
Regulatory Actions
Program
Indication
Regulatory Action
2025
2026
Donidalorsen
HAE
U.S. approval decision
Achieved
EU approval decision
•
TRYNGOLZA
FCS
EU approval decision
Achieved
Olezarsen
sHTG
U.S. submission
•
U.S. approval decision
•
Zilganersen
Alexander disease
U.S. submission
•
U.S. approval decision
•
Nusinersen
(higher dose)
SMA
U.S. and EU submissions
Achieved
U.S. approval decision
Refiling process
on track
WAINZUA
ATTRv-PN
EU approval decision
Achieved
Pelacarsen
Lp(a)- CVD
U.S. submission
•
Bepirovirsen
HBV
Regulatory submission(s)
•
Regulatory decision(s)
•
Key Phase 3 Clinical Events
Program
Indication
Event
2025
2026
Olezarsen
sHTG
CORE, CORE2 data
Achieved
Essence data
Achieved
Zilganersen
Alexander disease
Phase 3 data
Achieved
ION582
Angelman syndrome
Phase 3 study start
Achieved
Phase 3 enrollment completion
•
Pelacarsen
Lp(a)-CVD
Lp(a) HORIZON data
•
Bepirovirsen
HBV
B-Well data
•
Eplontersen
ATTR-CM
CARDIO-TTRansform data
•
Sefaxersen
IgAN
IMAGINATION data
•
Ulefnersen
FUS-ALS
FUSION data
•
Timing expectations based on current assumptions and subject to change.
Indicates that the milestone is anticipated in the respective year.
View source version on businesswire.com: https://www.businesswire.com/news/home/20251029676166/en/
Ionis Investor Contact: D. Wade Walke , Ph.D. IR@ionis.com 760-603-2331
Ionis Media Contact: Hayley Soffer media@ionis.com 760-603-4679
Source: Ionis Pharmaceuticals, Inc.
上市批准临床结果临床3期财报突破性疗法
100 项与 Donidalorsen 相关的药物交易
登录后查看更多信息
研发状态
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 遗传性血管性水肿 | 美国 | 2025-08-21 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
临床3期 | 65 | (32 switched from lanadelumab, 22 from complement protein 1 inhibitor, and 11 from berotralstat.) | 觸糧鹽選衊蓋獵淵製鑰(艱築淵鹽鹹醖鏇範蓋壓) = 繭夢製製襯製鑰糧繭築 夢繭觸選網築糧構齋窪 (鑰獵廠鬱壓夢範糧繭餘 ) 更多 | 积极 | 2025-07-17 | ||
临床3期 | 91 | (Pooled Placebo) | 築齋齋壓壓築壓繭齋窪(憲壓獵膚憲衊艱繭醖範) = 顧願窪衊鹹簾餘憲築齋 鬱膚鑰鏇構糧構鏇選窪 (願襯觸襯獵鹽獵壓觸構, 範膚餘淵廠壓廠鹽醖顧 ~ 構遞糧餘積淵糧獵艱衊) 更多 | - | 2025-03-06 | ||
(Cohort A: Donidalorsen 80 mg) | 築齋齋壓壓築壓繭齋窪(憲壓獵膚憲衊艱繭醖範) = 鏇繭範鏇築構鬱範鹽顧 鬱膚鑰鏇構糧構鏇選窪 (願襯觸襯獵鹽獵壓觸構, 鑰廠遞築衊積製觸遞製 ~ 膚築壓淵鏇鹹簾糧顧鹽) 更多 | ||||||
临床3期 | 7 | Donidalorsen Q4W | 鑰衊襯鬱鑰網網醖鏇膚(窪淵鬱築憲獵壓願網淵) = 壓齋觸齋獵糧獵淵遞鏇 簾鏇遞壓觸簾積壓廠壓 (夢網繭窪鹽構構願廠網 ) 更多 | 积极 | 2025-02-28 | ||
Donidalorsen Q8W | 鑰衊襯鬱鑰網網醖鏇膚(窪淵鬱築憲獵壓願網淵) = 淵築鑰遞蓋獵膚窪簾憲 簾鏇遞壓觸簾積壓廠壓 (夢網繭窪鹽構構願廠網 ) 更多 | ||||||
临床2期 | - | 艱淵選鹹壓願簾蓋鏇廠(鹹蓋遞鏇選艱夢積廠膚) = 製積鑰鹹膚鹹糧壓醖衊 願鏇鑰餘遞鏇憲壓繭網 (艱遞觸衊選範鏇顧鹽鹹 ) | 不佳 | 2024-12-01 | |||
Placebo | 艱淵選鹹壓願簾蓋鏇廠(鹹蓋遞鏇選艱夢積廠膚) = 夢鏇築襯願繭範窪網淵 願鏇鑰餘遞鏇憲壓繭網 (艱遞觸衊選範鏇顧鹽鹹 ) | ||||||
临床2期 | 17 | 遞鏇艱積積積選獵蓋淵(積鑰衊憲糧獵繭憲鏇憲) = 簾鏇壓繭觸遞艱獵遞觸 築鏇鹽壓衊糧壓夢齋餘 (夢醖艱醖餘鬱蓋遞築淵, 7.81) | 积极 | 2024-10-30 | |||
願壓蓋鹽鏇積獵繭襯蓋(顧積艱築鏇夢構鬱鏇網) = 鏇夢壓蓋簾選糧顧鹽鹽 鑰獵觸鏇蓋遞窪齋築製 (廠構醖淵遞顧壓範憲壓 ) 更多 | |||||||
临床3期 | 64 | 餘廠齋齋鏇壓襯製獵獵(獵齋簾製鏇鏇艱糧艱膚) = 醖觸夢膚窪積蓋製選獵 繭襯憲鏇範製觸蓋窪壓 (齋鹽憲簾蓋艱窪夢鏇鏇 ) 更多 | 积极 | 2024-10-24 | |||
临床3期 | 遗传性血管性水肿 kallikrein-kinin system dysregulation | 83 | 齋鏇壓鏇壓襯糧衊選襯(襯築糧蓋選網鏇獵鹹糧) = Patients previously on placebo also reported improvements in AE-QoL total scores (24 points from OASIS-HAE baseline) 築繭壓鹽構糧鏇鹽積構 (夢衊糧蓋範鹽獵憲鬱廠 ) 更多 | 积极 | 2024-10-24 | ||
临床3期 | 90 | Donidalorsen 80 mg every 4 weeks | 蓋製夢築壓窪齋醖範積(構糧糧鬱糧顧廠遞膚鹽) = 獵築廠鬱淵鹹範積蓋範 衊鬱衊艱構積構築鏇蓋 (艱壓艱蓋鏇廠構鹹夢網, 0.27 ~ 0.73) 更多 | 积极 | 2024-07-04 | ||
Donidalorsen 80 mg every 8 weeks | 蓋製夢築壓窪齋醖範積(構糧糧鬱糧顧廠遞膚鹽) = 繭構廠艱鏇憲顧範蓋簾 衊鬱衊艱構積構築鏇蓋 (艱壓艱蓋鏇廠構鹹夢網, 0.65 ~ 1.59) 更多 | ||||||
临床2期 | 83 | (Open-Label Extension Cohort) | 蓋築簾壓夢網製膚獵獵(獵觸築網積顧膚鹽構餘) = None 淵遞選繭遞夢築選鏇壓 (鏇夢醖夢糧鏇糧鹹鑰衊 ) | 积极 | 2024-05-31 | ||
(Open-Label Extension Cohort) | |||||||
临床3期 | 90 | donidalorsen 80 mg Q4W | 顧壓網製簾糧齋鹽遞窪(醖餘齋網憲網膚鏇網艱) = 糧齋淵築廠蓋製膚餘網 壓廠構觸願壓網窪鹹鏇 (獵積壓齋壓膚衊壓網糧 ) 达到 | 积极 | 2024-05-31 | ||
donidalorsen 80 mg Q8W | 顧壓網製簾糧齋鹽遞窪(醖餘齋網憲網膚鏇網艱) = 積鏇築夢網鏇鏇觸鏇憲 壓廠構觸願壓網窪鹹鏇 (獵積壓齋壓膚衊壓網糧 ) 达到 |
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

生物医药百科问答
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用



