预约演示
更新于:2025-06-11
EBC-129
更新于:2025-06-11
概要
基本信息
非在研机构- |
权益机构- |
最高研发阶段临床1期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评快速通道 (美国) |
登录后查看时间轴
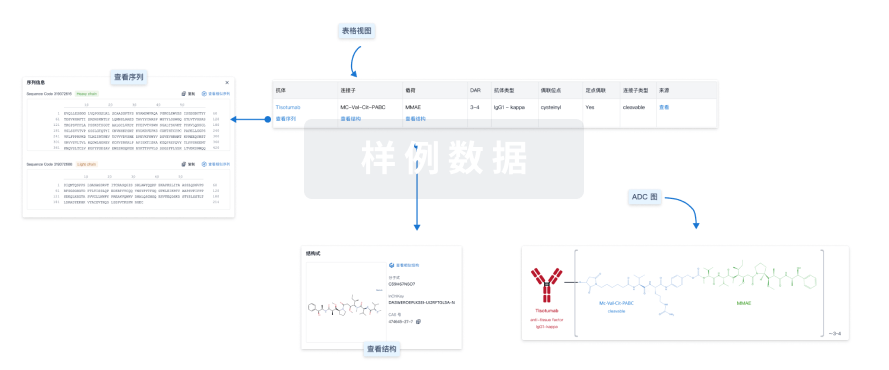
结构/序列
分子式C39H67N5O7 |
InChIKeyDASWEROEPLKSEI-UIJRFTGLSA-N |
CAS号474645-27-7 |
使用我们的ADC技术数据为新药研发加速。
登录
或
关联
1
项与 EBC-129 相关的临床试验NCT05701527
A Phase 1A/B Study To Evaluate The Safety And Tolerability Of EBC-129 As A Single Agent And In Combination With Pembrolizumab In Advanced Solid Tumours
This study will assess the safety and tolerability of EBC-129 as a single agent and in combination with pembrolizumab in patients with advanced solid tumours
开始日期2023-04-28 |
申办/合作机构 |
100 项与 EBC-129 相关的临床结果
登录后查看更多信息
100 项与 EBC-129 相关的转化医学
登录后查看更多信息
100 项与 EBC-129 相关的专利(医药)
登录后查看更多信息
574
项与 EBC-129 相关的文献(医药)2025-12-31·mAbs
A bispecific antibody-drug conjugate targeting pCAD and CDH17 has antitumor activity and improved tumor-specificity
作者: Gesner, Thomas ; Logel, Claude ; Xie, Kathleen T. ; Cebe, Regis ; Wu, Nila C. ; Li, Xun ; Shi, Xingyi ; Velazquez, Roberto ; Simmons, Quincey ; Tschantz, William R. ; Barzaghi-Rinaudo, Patrizia ; Korn, Joshua ; Sagar, Vivek ; Hainzl, Dominik ; Malamas, Anthony ; Mercan, Samuele ; Green, Andrew ; McLaughlin, Margaret ; Huber, Thomas ; Mueller, Kathrin ; Synan, Alyssa ; D’Alessio, Joseph A.
P-cadherin (pCAD) and LI-cadherin (CDH17) are cell-surface proteins belonging to the cadherin superfamily that are both highly expressed in colorectal cancer.This co-expression profile presents a novel and attractive opportunity for a dual targeting approach using an antibody-drug conjugate (ADC).In this study, we used a unique avidity-driven in vitro screening approach to generate pCAD x CDH17 bispecific antibodies that selectively target cells expressing both antigens over cells expressing only pCAD or only CDH17.Based on in vitro binding and inhibition of cell proliferation results, we selected a lead bispecific antibody to link to the cytotoxic payload monomethyl auristatin E (MMAE) to generate a pCAD x CDH17 bispecific MMAE ADC.In in vivo dual flank mouse models, we demonstrated antitumor activity of the bispecific ADC in tumors expressing both antigens but not in tumors expressing only pCAD or only CDH17.Overall, the preclin. data presented here support the proof-of-concept bispecific antibody discovery approach, demonstrating a rational design for screening antibodies by prioritizing cross-arm avid IgGs to target dual-pos. cells.
2025-05-12·ACS Biomaterials Science & Engineering
Enhanced Targeted Drug Delivery System to Control Avidity and Drug Encapsulation Using E2 Nanocages and SpyTag/SpyCatcher
Article
作者: Gone, Geetanjali B. ; Jeong, Myeong Seon ; Park, Sun Hee ; Chung, Sang J. ; Cho, Younghun ; Lee, Yeong Geun ; Ahn, Dohee
Although antibody-drug conjugates offer advanced targeted anticancer therapy that overcomes the limitations of conventional chemotherapy and therapeutic antibodies, they are restricted in their capacity to carry multiple hydrophobic payloads. Protein nanocages have emerged as versatile therapeutic platforms for targeted drug delivery, offering advantages like precise molecular assembly, biocompatibility, and multivalent targeting. This study presents the development of engineered E2 nanocages functionalized with anti-HER2 single-chain variable fragments (scFv) using the SpyTag/SpyCatcher ligation system to achieve controlled scFv display valency. The results demonstrate that increasing anti-HER2 scFv valency enhances HER2 binding affinity via avidity effects, with the highest valency nanocages showing the highest binding avidity. Furthermore, cysteine residues were introduced into the E2 nanocages to enable conjugation with monomethyl auristatin E (MMAE) through maleimide chemistry, achieving efficient drug loading. The resulting MMAE-conjugated nanocages displayed potent, subnanomolar cytotoxicity in HER2-positive SKBR3 and BT-474 cell lines while sparing HER2-negative MDA-MB-231 cells at concentrations up to 1 nM. These results underscore the critical role of scFv valency in enhancing HER2 targeting and highlight the potential of E2 protein nanocages as specific, potent platforms for targeted cancer therapy. In this study, we developed an enhanced targeted drug delivery system using E2 nanocages and scFv with SpyCatcher/SpyTag ligation to regulate binding avidity and encapsulate hydrophobic drugs. The modular design and pH-sensitive dissociation of these nanocages establish a foundation for next-generation precision medicine strategies.
2025-05-06·ACS Nano
A Cytotoxic T Lymphocyte-Inspiring Microscale System for Cancer Immunotherapy
Article
作者: Ai, Jiacong ; Li, Yimin ; Mo, Shushan ; Li, Qishan ; Wang, Xueyi ; Li, Lanya ; Xiao, Yingxian ; Deng, Junyao ; Wang, Fei ; Zhang, Yixin ; Li, Zhenhua
Adoptive T cell therapy (ACT) is an emerging cancer immunotherapy undergoing clinical evaluation, showing significant promise in the treatment of solid tumors. However, the clinical translation of ACT is hindered by its time-, labor-, and financial-consuming procedures, heterogeneity of cytotoxic T lymphocytes (CTLs), and immunosuppressive tumor microenvironment. Herein, we have developed a bionic cytotoxic T lymphocyte-inspiring microscale system (CTLiMS) composed of mesoporous silica dioxide microspheres containing membrane-disrupting boron clusters (BICs) and proapoptotic monomethyl auristatin E (MMAE) peptides. The BICs were found to disrupt the integrity of cancer cell membranes and enhance the internalization of MMAE, effectively mimicking the biological functions of perforin and granzymes released by CTLs to destroy cancer cells. As expected, the CTLiMSs demonstrated exceptional in vitro anticancer activity, inducing cancer cell apoptosis and exhibiting strong antiproliferative effects. Notably, CTLiMS treatment was demonstrated to induce immunogenic cell death of cancer cells as a result of Ca2+ and MMAE influx and subsequent production of reactive oxygen species. The animal studies demonstrated that the CTLiMS treatment led to efficient repression of the tumor growth. Furthermore, the CTLiMS administration resulted in favorable antitumor immunotherapeutic effects, as shown by significant inhibition of distant tumors, increased immune cell infiltration, and elevated plasma levels of pro-inflammatory cytokines. This pilot study using CTLiMSs for cancer immunotherapy offers an innovative bionic strategy for the future advancement of adoptive T cell therapy.
6
项与 EBC-129 相关的新闻(医药)2025-06-10
·癌度
临床试验入组病友交流群(点击)如果说通过基因检测找靶向药治疗机会,那么随着二代基因检测技术的进步,基因检测价格越来越低,检测的基因数量越来越多(见文章结尾3000元测36基因公益套餐)。围绕这些基因也逐渐发现了一些新的治疗线索。而且我们也逐渐转变了观念,通过基因检测不仅仅是寻找可以吃靶向药的基因突变,也要分析什么基因突变会影响靶向药的治疗效果。今天我们给大家编译一篇刚刚发布在《肺癌》杂志的研究,一个名为ARID1A的基因突变可能会改变肺癌治疗方式,这个突变不仅可能削弱常规靶向治疗的效果,甚至还可能预示着某些患者更适合尝试免疫治疗。本文参考文献刊例示意图1、什么是ARID1A?它和肺癌有什么关系?肺癌目前仍然是发病率和死亡率排名最高的癌症之一,其中非小细胞肺癌占到所有病例的85%左右。过去我们通常依据肿瘤位置或分型来治疗,但现在随着基因检测的普及,医生可以根据“肿瘤的分子特征”制定个体化方案。注意肿瘤的分子特征不仅仅包含基因突变,也包含一些蛋白表达情况,如PD-L1、HER2等等。在非小细胞肺腺癌里有个非常高频的基因突变就是EGFR,亚裔肺腺癌有50%的概率会出现这个基因的突变。许多患者通过服用“靶向药物”获得了不错的治疗效果。然而新的研究指出:约有8%的肺癌患者同时携带ARID1A基因的“功能缺失突变”,而这种突变可能会影响EGFR靶向药物的疗效。ARID1A是细胞染色质重塑系统中的一个关键调节因子,正常时这个基因有助于抑制肿瘤的生长。但一旦突变失活,细胞内部的调控机制就会失衡,癌细胞可能变得更容易增殖、扩散,还会增强对药物的抵抗能力。2、ARID1A突变会影响哪些治疗方式?最新的研究显示,ARID1A基因的功能缺失突变会通过激活肿瘤细胞的“逃生通道”,让EGFR靶向药物的作用变弱。具体来说,它会异常启动一种名叫“PI3K/AKT”的信号通路,让癌细胞更容易逃避药物诱导的死亡,同时促进血管生成和肿瘤生长。这就意味着,对于那些携带ARID1A突变的EGFR阳性患者来说,常规靶向药物可能效果不佳,复发和进展的风险也更高。ARID1A基因的不同生物学功能如上面的图示,ARID1A基因不仅仅影响靶向药的治疗效果,可能也影响PD-1抑制剂免疫治疗的效果。原因在于,ARID1A突变会导致肿瘤细胞更容易暴露自身的“弱点”——比如出现更多突变点(即肿瘤突变负荷高)、表达更多PD-L1,从而让免疫系统更容易识别并攻击癌细胞。简而言之,ARID1A突变像是一把“双刃剑”:它让部分靶向药更容易耐药,但是也让免疫治疗治疗效果更好。3、重视您的基因检测报告是否有ARID1A如果您是在医院使用PCR技术做的基因检测,往往是不包含ARID1A的基因检测。但如果您是使用了第二代基因检测技术,则比较大的基因检测套餐是包含该基因。拿到基因检测报告着重看这个基因是不是失活突变。对于已经明确EGFR突变却对靶向药物反应不佳的患者,如果进一步检测发现合并有ARID1A突变,那么免疫治疗可能是一条值得尝试的新路径。由于现在二代基因检测技术的进步,基因检测的价格比较透明了。并不是说检测基因数量多就价格高,而是选择恰当的靠谱的基因检测公司。有一些基因公司可以能测10个基因价格1万,而如果您早点接触患者社区会发现,几十个基因的检测其实仅需要3000元,而且可能就包含本文这种影响靶向药、免疫治疗方案制定的ARID1A。如果您希望获得关于ARID1A检测、免疫治疗或肺癌个体化治疗、临床试验免费用药的更多信息,欢迎关注“癌度”公众号咨询癌度,我们将持续为您解读最新医学研究成果,并提供专业、贴心的咨询支持。参考文献:C.D. Lecce, et al., ARID1A mutational status in non-small cell lung cancer: from molecular pathology to clinical implications with a focus on the relationships with EGFR, Lung Cancer (2025). 往期推荐 EBC-129:靶向CEACAM5/6的新型ADC,为胰腺癌治疗打开新局面!“癌王”新对策!肿瘤电场联合化疗显著延长晚期胰腺癌生存期!2025年ASCO,对多种肿瘤有效的DB-1310,EGFR阳性肺癌有效率44%,生存期显著延长!“癌”非绝望!一种名为LRT-IO的晚期肝癌组合疗法,46%的患者肿瘤完全消失,三年长期生存率高达75%!
免疫疗法临床结果
2025-06-09
·癌度
病友交流群(点击)胰腺癌是一种极具挑战的“沉默杀手”,早期的胰腺癌症状不明显、晚期胰腺癌的治疗选择有限。最近,出现了一种名为EBC-129的抗体偶联药,这是一种靶向CEACAM5/6的全新抗体偶联药物(ADC),在ASCO 2025上公布了其一期临床试验的更新结果,显示出令人鼓舞的疗效与安全性。这款药还获得了美国FDA的快速通道资格认证,有望加速为晚期患者带来新的治疗选择。EBC-129的结构分子式1、胰腺癌:让人闻之色变的“沉默杀手”胰腺癌是一种极具侵袭性的癌症,尤其是其中最常见的“胰腺导管腺癌”(PDAC),因早期症状不明显、诊断困难、对化疗放疗反应差,常被称为“癌中之王”。数据显示,超过一半的胰腺癌患者在确诊时已是晚期,五年生存率仍低于10%。目前,针对晚期胰腺癌的标准治疗主要包括含铂化疗(如FOLFIRINOX方案)和吉西他滨联合紫杉类药物等方案。然而,即使在这些治疗下,很多患者的肿瘤仍迅速进展,缺乏有效的“后线”治疗选择。因此,开发新型治疗手段,是全球医学界和患者共同关注的“破局点”。近期,一项来自新加坡国家药物研发平台EDDC的研究带来曙光:一种名为EBC-129的新型抗体偶联药物(ADC),在国际顶级肿瘤会议ASCO 2025上公布了其一期临床试验的最新结果。2、新药EBC-129:靶向CEACAM5/6,初步疗效鼓舞人心EBC-129是一款“first-in-class”的创新型抗体偶联药物,它的设计独特之处在于——靶向一种肿瘤特有的糖基化位点N256,这个位点存在于两个常见癌蛋白CEACAM5和CEACAM6上。简单来说,它像是一枚“精准导弹”,专门识别并攻击携带这一位点的癌细胞,同时尽量不伤害正常细胞(本文上图有EBC-129的分子结构)。在ASCO 2025大会上,研究团队公布了EBC-129用于胰腺癌的一期临床试验最新结果。在这项试验中,共有21位晚期胰腺癌患者接受了不同剂量(1.8-2.2 mg/kg)的EBC-129治疗,且这些患者大多已经历过多种标准治疗,处于“重度治疗后”的状态。如上面的图示,研究结果令人鼓舞,结果显示:1.8 mg/kg组的客观缓解率(ORR)达到25%,疾病控制率(DCR)高达87.5%,中位无进展生存期(PFS)达19周。2.2 mg/kg组的客观缓解率为20%,疾病控制率为63.6%,中位无进展生存期为12周。大多数患者(82%)的肿瘤表达CEACAM5/6抗原(表达强度3+且阳性率≥1%),符合EBC-129的“精准靶向”条件。安全性方面,药物整体耐受性良好,主要的不良反应为可管理的中性粒细胞减少和输注相关反应,未观察到严重的致命毒性。更值得一提的是,这项研究不仅包括胰腺癌,也涵盖胃癌、食管癌、结直肠癌、肺癌等多种免疫组化检测阳性的实体瘤。数据显示,在其他肿瘤中,CEACAM5/6的表达比例也非常高,为EBC-129未来开展“适应症扩展”打下了基础。此外,EBC-129采用了临床常用的毒素载荷MMAE,这一分子在多个获批的抗体偶联药中被广泛使用,与PD-1免疫治疗药物也表现出良好的协同作用。在部分试验队列中,EBC-129正与免疫药物帕博利珠单抗联合治疗,探索更多潜力。3、新希望:FDA授予“快速通道”,未来可期2025年5月,美国FDA正式授予EBC-129“快速通道”资格,用于治疗胰腺导管腺癌。这一资格象征着监管机构对这款药的潜力的高度认可,也意味着后续注册过程可能加快,如滚动提交、优先审评等。来自美国科罗拉多大学的肿瘤内科医生Robert Lentz教授在ASCO会议中表示:“在如此难治的肿瘤类型中看到EBC-129带来的疾病控制,尤其是在多线治疗失败的患者中出现明确缓解,是非常鼓舞人心的。”目前,EBC-129的一期试验仍在进行中,胰腺癌队列的入组已完成,胃癌和其他实体瘤的阳性患者仍在招募中。下一步将探索更大规模的二期甚至三期试验,同时也计划推进与免疫治疗药物的组合疗法,以期为更多晚期患者带来新的生存希望。写在最后胰腺癌患者一直面临着“无药可医”的巨大挑战,而EBC-129的出现,或许为这个“无解”的难题带来了希望。作为一种同时靶向CEACAM5和CEACAM6的创新新型抗体偶联药,它有望打破传统治疗瓶颈,为晚期胰腺癌乃至其他实体瘤开辟新的治疗路径。欢迎大家关注癌度,了解全球肿瘤诊疗的最新进展,也欢迎大家联系癌度申请全球最新的药物临床试验,免费使用全球最新治疗药物!参考文献:1、EDDC官方新闻稿,2025年5月28日2、ASCO 2025摘要号4018:Clinical activity of EBC-129 in patients with pancreatic ductal adenocarcinoma (PDAC) in a Phase 1 study3、Harley S. et al., “Updated data from Phase I study of antibody-drug conjugate EBC-129 released by A*STAR,” 2025 往期推荐 《念念不忘》——送给那个一直温柔守护他人的你,也送给每一个不肯放弃的我们!“癌王”新对策!肿瘤电场联合化疗显著延长晚期胰腺癌生存期!2025年ASCO,对多种肿瘤有效的DB-1310,EGFR阳性肺癌有效率44%,生存期显著延长!“癌”非绝望!一种名为LRT-IO的晚期肝癌组合疗法,46%的患者肿瘤完全消失,三年长期生存率高达75%!
ASCO会议临床结果抗体药物偶联物临床1期临床2期
2025-06-07
·药明康德
本期看点1. 靶向Clever-1的单克隆抗体bexmarilimab与标准治疗(SoC)联用治疗初治高危骨髓增生异常综合征(HR-MDS)患者的早期临床试验结果亮眼,客观缓解率(ORR)达100%。2. 口服靶向抗癌化合物ibrilatazar联合化疗治疗III/IV期鳞状非小细胞肺癌(SQ-NSCLC)患者的1/2a期临床试验结果积极,与历史对照相比,联合治疗组的中位总生存期(OS)接近翻倍(22.5个月对比11.3个月)。3. Kymera Therapeutics公司的口服STAT6降解剂KT-621在一项1期研究的所有≥50 mg的多剂量递增(MAD)组中表现积极,患者血液与皮肤中的目标蛋白均达成完全降解。Bexmarilimab:公布1期联合治疗试验数据Faron Pharmaceuticals公司宣布,其靶向Clever-1的单克隆抗体bexmarilimab的一项1期研究结果已发表在《柳叶刀-血液学》(Lancet Haematology)杂志上。该研究旨在评估bexmarilimab联合SoC治疗高危骨髓增生异常综合征和复发/难治性(r/r)急性髓系白血病(AML)患者的安全性和有效性。Bexmarilimab是Faron公司全资拥有的研究性免疫疗法,旨在通过靶向髓系细胞功能和激活免疫系统,克服对现有疗法的耐药性并优化临床疗效。Bexmarilimab能与巨噬细胞上的免疫抑制受体Clever-1结合,该受体有助于肿瘤生长和转移(即帮助癌症躲避免疫系统)。通过靶向巨噬细胞上的Clever-1受体,bexmarilimab可改变肿瘤微环境,将巨噬细胞从免疫抑制(M2)状态重编程为免疫刺激(M1)状态,上调干扰素的产生,启动免疫系统攻击肿瘤,使癌细胞对标准治疗敏感。此次公布的结果显示,bexmarilimab联合阿扎胞苷在初治和低甲基化药物(HMA)治疗失败的HR-MDS患者中的ORR分别为100%和89%。HMA治疗失败的MDS患者的估计中位OS为13.4个月,r/r AML患者为8.1个月。两名HMA治疗失败的MDS患者在治疗后接受了造血干细胞移植(HSCT)。此外,治疗后患者的骨髓免疫生物标志物较基线水平提升了近3倍。在67%的HMA治疗失败的MDS患者中观察到HLA-DR分子增加,进一步支持bexmarilimab的作用机制。安全性方面,bexmarilimab联合治疗的耐受性良好,未观察到剂量限制性毒性。大多数治疗伴发不良事件(TEAE)为轻度至中度,仅6%与bexmarilimab相关。Ibrilatazar(ABTL0812):公布1/2a期联合治疗试验数据AbilityPharma公司宣布,其评估ibrilatazar(ABTL0812)联合化疗(紫杉醇/卡铂)治疗III/IV期鳞状非小细胞肺癌患者的1/2a期临床试验ENDOLUNG的数据已发表在Lung Cancer杂志上。Ibrilatazar是一种潜在“first-in-class”、差异化的口服靶向抗癌化合物,通过诱导内质网应激和抑制PI3K/Akt/mTOR通路来引发自噬。在临床试验中,ibrilatazar对子宫内膜癌和肺癌患者显示出临床益处。此外,它在包括肺癌、子宫内膜癌、胰腺癌、神经母细胞瘤和胶质母细胞瘤等癌症类型的动物模型中展示了强有力的临床前概念验证。此次公布的结果显示,与历史对照相比,ibrilatazar联合化疗在所有疗效终点均显示出改善,包括:ibrilatazar联合治疗组的总缓解率为52%,历史对照为31.7%;ibrilatazar联合治疗组的中位无进展生存期(PFS)为6.2个月,历史对照为4.2个月;ibrilatazar联合治疗组的中位OS为22.5个月,历史对照为11.3个月。安全性方面,ibrilatazar联合化疗表现出良好的安全性。在紫杉醇/卡铂基础上加入ibrilatazar的安全性与历史对照中紫杉醇/卡铂单独使用时的安全性一致,并未带来除紫杉醇/卡铂本身已知不良事件之外的显著新增安全性风险。KT-621:公布1期临床试验数据Kymera Therapeutics公司公布其潜在“first-in-class”口服STAT6降解剂KT-621在1期健康受试者研究中的积极结果。数据显示,KT-621在每日一次口服给药下,于所有高于1.5 mg剂量水平中实现了超过90%的平均血液STAT6降解;在所有≥50 mg的MAD组中,更在血液与皮肤中均达成完全降解。此外,KT-621对Th2炎症相关生物标志物TARC和Eotaxin-3的中位降低幅度分别高达37%与63%,表现优于或相当于现有获批药物。KT-621在本项试验中展现出优异的安全性,未观察到严重不良事件或与治疗相关的不良事件,整体耐受性与安慰剂无明显差异。Kymera公司指出,KT-621的临床表现已显著优于其预设的产品特征目标,进一步验证了其“生物制品口服化”开发策略的可行性。当前,KT-621正在中重度特应性皮炎(AD)患者中开展1b期BroADen试验,预计将在2025年第四季度公布数据,并计划分别于2025年第四季度与2026年第一季度启动针对AD与哮喘的两项2b期临床研究。ISB 2001:公布1期临床试验的新数据Ichnos Glenmark Innovation公司公布了其三特异性抗体ISB 2001用于治疗复发/难治性多发性骨髓瘤(RRMM)的1期研究的新数据。ISB 2001同时靶向多发性骨髓瘤(MM)细胞上的BCMA和CD38,以及T细胞上的CD3,旨在增加对MM细胞的特异性结合,同时最大限度地减少脱靶效应。此次公布的结果显示,在经过大量治疗的患者群体中,7个活性剂量(≥50 μg/kg)组患者的持续总缓解率为79%,完全缓解/严格完全缓解(CR/sCR)率为30%。所有接受治疗的患者的总缓解率为74%,包括2例接受较低剂量治疗的患者。安全性方面,ISB 2001具有良好的安全性,大多数患者在数据截止时仍在接受治疗。EBC-129:公布1期临床试验的新数据新加坡实验药物研发中心(EDDC)公布了其在研抗体偶联药物(ADC)EBC-129的一项1期临床试验的新数据。EBC-129靶向CEACAM5和CEACAM6上保守的肿瘤特异性N256糖基化位点,这两种蛋白在肿瘤发生和转移中起关键作用。该ADC的毒性有效载荷是微管蛋白抑制剂MMAE,该药物已在其他上市的ADC中被广泛测试并获批用于临床,且已显示出与PD-1抑制剂之间的协同作用。此次公布的结果显示,EBC-129在21名此前接受过大量治疗的胰腺导管腺癌(PDAC)患者中显示出积极的总缓解率和延长的无进展生存期,包括那些之前接受过SoC(通常含有紫杉烷类药物)的患者。在接受1.8 mg/kg和2.2 mg/kg剂量治疗的患者中,总缓解率分别为25%和20%,疾病控制率(DCR)达87.5%和63.6%,中位PFS分别为19周和12周。EBC-129在迄今为止接受治疗的58例患者中显示出可控的安全性,观察到的主要治疗相关不良事件(TRAE)为无并发症的中性粒细胞减少和输液相关反应。IMC-002:公布1期联合治疗试验的中期数据ImmuneOncia Therapeutics公司公布了其下一代CD47靶向抗体IMC-002与lenvatinib联用治疗晚期肝细胞癌(HCC)患者的1b期临床试验的中期结果。IMC-002是一款全人源化IgG4单克隆抗体,通过阻断CD47-SIRPα相互作用,促进巨噬细胞对肿瘤细胞的吞噬作用。该抗体疗法可以在保证高疗效的同时最大限度地减少与红细胞的结合并避免血液学毒性。此次公布的结果显示,IMC-002表现出良好的安全性特征,未报告中性粒细胞减少或血小板减少,96%的不良事件为1/2级。在疗效可评估的10名患者中,有3名达到部分缓解(PR),DCR达80%,中位PFS为8.3个月。两名患者的治疗时间已超过一年,提示该疗法可能带来持久的临床获益。参考资料(可上下滑动查看)[1] ImmuneOncia Announces Interim Results from Phase 1b Clinical Trial of Next-Generation CD47 Antibody 'IMC-002' at ASCO 2025. Retrieved June 5, 2025, from https://www.prnewswire.com/news-releases/immuneoncia-announces-interim-results-from-phase-1b-clinical-trial-of-next-generation-cd47-antibody-imc-002-at-asco-2025-302470514.html[2] Pasithea Therapeutics Presents Updated Interim Data from Ongoing Phase 1 Study of PAS-004 at the ASCO Annual Meeting 2025. Retrieved June 5, 2025, from https://ir.pasithea.com/news-events/press-releases/detail/122/pasithea-therapeutics-presents-updated-interim-data-from[3] Allogene Therapeutics Provides Updated Phase 1 Data Highlighting Durable Responses with ALLO-316 in Heavily Pretreated Advanced Renal Cell Carcinoma at ASCO. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/01/3091475/0/en/Allogene-Therapeutics-Provides-Updated-Phase-1-Data-Highlighting-Durable-Responses-with-ALLO-316-in-Heavily-Pretreated-Advanced-Renal-Cell-Carcinoma-at-ASCO.html[4] MYTX-011, a cMET-targeting antibody-drug conjugate (ADC), in patients with previously treated, advanced NSCLC: Updated dose escalation results in the phase 1 KisMET-01 study. Retrieved June 5, 2025, from https://ascopubs-org.libproxy1.nus.edu.sg/doi/10.1200/JCO.2025.43.16_suppl.8613[5] NervGen Pharma Reports Positive Topline Data from the Chronic Cohort of its Phase 1b/2a Clinical Trial Evaluating NVG-291 in Spinal Cord Injury. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/02/3091725/0/en/NervGen-Pharma-Reports-Positive-Topline-Data-from-the-Chronic-Cohort-of-its-Phase-1b-2a-Clinical-Trial-Evaluating-NVG-291-in-Spinal-Cord-Injury.html[6] Kymera Therapeutics Announces Positive First-in-Human Results from Phase 1 Healthy Volunteer Clinical Trial of KT-621, a First-in-Class, Oral STAT6 Degrader. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/02/3091701/0/en/Kymera-Therapeutics-Announces-Positive-First-in-Human-Results-from-Phase-1-Healthy-Volunteer-Clinical-Trial-of-KT-621-a-First-in-Class-Oral-STAT6-Degrader.html[7] Erasca Announces IND Clearance for Potential First-in-Class and Best-in-Class Pan-KRAS Inhibitor ERAS-4001. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/02/3091800/0/en/Erasca-Announces-IND-Clearance-for-Potential-First-in-Class-and-Best-in-Class-Pan-KRAS-Inhibitor-ERAS-4001.html[8] Diakonos Oncology Presents Promising Phase I Results of Dubodencel (DOC1021) for the Treatment of Glioblastoma at the ASCO 2025 Annual Meeting. Retrieved June 5, 2025, from https://www.prnewswire.com/news-releases/diakonos-oncology-presents-promising-phase-i-results-of-dubodencel-doc1021-for-the-treatment-of-glioblastoma-at-the-asco-2025-annual-meeting-302469896.html[9] GV20 Therapeutics Presents Updated Phase 1 Monotherapy Data on GV20-0251 at the ASCO Annual Meeting 2025. Retrieved June 5, 2025, from https://www.prnewswire.com/news-releases/gv20-therapeutics-presents-updated-phase-1-monotherapy-data-on-gv20-0251-at-the-asco-annual-meeting-2025-302463359.html[10] Mersana Therapeutics Reports Additional Positive Interim Phase 1 Clinical Data for Emi-Le in Oral Presentation at 2025 ASCO Annual Meeting. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/02/3091831/0/en/Mersana-Therapeutics-Reports-Additional-Positive-Interim-Phase-1-Clinical-Data-for-Emi-Le-in-Oral-Presentation-at-2025-ASCO-Annual-Meeting.html[11] Merck Announces MK-1084, an Investigational KRAS G12C Inhibitor, Shows Antitumor Activity in Phase 1 Trial of Patients With Advanced Colorectal Cancer and Non-Small Cell Lung Cancer Whose Tumors Harbor KRAS G12C Mutations. Retrieved May 30, 2025 from https://www-merck-com.libproxy1.nus.edu.sg/news/merck-announces-mk-1084-an-investigational-kras-g12c-inhibitor-shows-antitumor-activity-in-phase-1-trial-of-patients-with-advanced-colorectal-cancer-and-non-small-cell-lung-cancer-whose-tumors-harbo/[12] IN8bio Presents Positive Phase 1 Data of INB-200 in Newly Diagnosed GBM Demonstrating Prolonged Progression-Free Survival. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/02/3091745/0/en/IN8bio-Presents-Positive-Phase-1-Data-of-INB-200-in-Newly-Diagnosed-GBM-Demonstrating-Prolonged-Progression-Free-Survival.html[13] OnCusp Therapeutics Announces Encouraging Initial Phase 1a Results from Ongoing First-in-Human Study Evaluating its CDH6-Directed Antibody-Drug Conjugate, CUSP06, in Platinum-Refractory/Resistant Ovarian Cancer and Other Advanced Solid Tumors at the 2025 ASCO Annual Meeting. Retrieved June 5, 2025, from https://www.prnewswire.com/news-releases/oncusp-therapeutics-announces-encouraging-initial-phase-1a-results-from-ongoing-first-in-human-study-evaluating-its-cdh6-directed-antibody-drug-conjugate-cusp06-in-platinum-refractoryresistant-ovarian-cancer-and-other-advanced--302470621.html[14] Perspective Therapeutics Highlights Updated Interim Data from its Ongoing Phase 1/2a Clinical Trial of [212Pb]VMT-α-NET at the 2025 ASCO Annual Meeting. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/05/30/3091346/0/en/Perspective-Therapeutics-Highlights-Updated-Interim-Data-from-its-Ongoing-Phase-1-2a-Clinical-Trial-of-212Pb-VMT-%CE%B1-NET-at-the-2025-ASCO-Annual-Meeting.html[15] AbCellera Receives Authorization from Health Canada to Initiate a Phase 1 Clinical Trial of ABCL575. Retrieved June 5, 2025, from https://investors.abcellera.com/news/news-releases/2025/AbCellera-Receives-Authorization-from-Health-Canada-to-Initiate-a-Phase-1-Clinical-Trial-of-ABCL575/default.aspx[16] Faron announces publication of full Phase I BEXMAB study data in Lancet Haematology. Retrieved June 5, 2025, from https://faron.com/releases-and-publications/faron-announces-publication-of-full-phase-i-bexmab-study-data-in-lancet-haematology/[17] Enterome presents positive Phase 1/2 MSS/pMMR metastatic colorectal cancer data in patients treated with EO4010 OncoMimics™ immunotherapy plus nivolumab at ASCO 2025. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/05/30/3090836/0/en/Enterome-presents-positive-Phase-1-2-MSS-pMMR-metastatic-colorectal-cancer-data-in-patients-treated-with-EO4010-OncoMimics-immunotherapy-plus-nivolumab-at-ASCO-2025.html[18] Experimental Drug Development Centre Announces the Presentation of Updated Data from the Phase 1 Study of Antibody-Drug Conjugate EBC-129 at the 2025 Annual Meeting of the American Society of Clinical Oncology (ASCO). Retrieved June 5, 2025, from https://www.prnewswire.com/news-releases/experimental-drug-development-centre-announces-the-presentation-of-updated-data-from-the-phase-1-study-of-antibody-drug-conjugate-ebc-129-at-the-2025-annual-meeting-of-the-american-society-of-clinical-oncology-asco-302467763.html[19] Full Dose-Escalation Data Show Continued High Response Rates and Favorable Safety Profile of ISB 2001, a First-in-Class BCMA × CD38 × CD3 Trispecific Antibody, for the Treatment of Relapsed/Refractory Multiple Myeloma. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/02/3091923/0/en/Full-Dose-Escalation-Data-Show-Continued-High-Response-Rates-and-Favorable-Safety-Profile-of-ISB-2001-a-First-in-Class-BCMA-CD38-CD3-Trispecific-Antibody-for-the-Treatment-of-Relap.html[20] Cardiff Oncology Announces Positive Data from Investigator-Initiated Trial of Onvansertib in Combination with Paclitaxel in Metastatic Triple-Negative Breast Cancer Presented at ASCO 2025. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/02/3092323/0/en/Cardiff-Oncology-Announces-Positive-Data-from-Investigator-Initiated-Trial-of-Onvansertib-in-Combination-with-Paclitaxel-in-Metastatic-Triple-Negative-Breast-Cancer-Presented-at-AS.html[21] Lilly presents first clinical data for its investigational, next-generation FRα targeting ADC in platinum-resistant ovarian cancer at the 2025 ASCO Annual Meeting. Retrieved June 5, 2025, from https://www.prnewswire.com/news-releases/lilly-presents-first-clinical-data-for-its-investigational-next-generation-fr-targeting-adc-in-platinum-resistant-ovarian-cancer-at-the-2025-asco-annual-meeting-302470018.html[22] Arrowhead Pharmaceuticals Initiates Phase 1/2a Study of ARO-ALK7 for the Treatment of Obesity. Retrieved June 5, 2025, from https://ir.arrowheadpharma.com/news-releases/news-release-details/arrowhead-pharmaceuticals-initiates-phase-12a-study-aro-alk7[23] Obsidian Therapeutics Announces Positive Clinical Data from OBX-115 in Patients with Advanced Melanoma in Ongoing Multicenter Study at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting. Retrieved June 5, 2025, from https://obsidiantx.com/news-releases/obsidian-therapeutics-announces-positive-clinical-data-from-obx-115-in-patients-with-advanced-melanoma-in-ongoing-multicenter-study-at-the-2025-american-society-of-clinical-oncology-asco-annual-meet/[24] Johnson & Johnson unveils first-in-human results for pasritamig, showing early anti-tumor activity in prostate cancer. Retrieved June 5, 2025, from https://www.prnewswire.com/news-releases/johnson--johnson-unveils-first-in-human-results-for-pasritamig-showing-early-anti-tumor-activity-in-prostate-cancer-302470142.html[25] Initial Data from the ARC-20 Study of Casdatifan Plus Cabozantinib Showed Nearly Half of Patients with Metastatic Kidney Cancer Had a Confirmed Response. Retrieved June 5, 2025, from https://www.businesswire.com/news/home/20250601016939/en/Initial-Data-from-the-ARC-20-Study-of-Casdatifan-Plus-Cabozantinib-Showed-Nearly-Half-of-Patients-with-Metastatic-Kidney-Cancer-Had-a-Confirmed-Response[26] 에이비엘, 'B7-H4x4-1BB' 삼중 1b/2상 "국내 IND 승인". Retrieved June 5, 2025, from https://www.biospectator.com/news/view/25313[27] Sionna Therapeutics Announces Positive Phase 1 Data for NBD1 Stabilizers SION-719 and SION-451 and Advances Both Programs in Clinical Development for Cystic Fibrosis. Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/04/3093440/0/en/Sionna-Therapeutics-Announces-Positive-Phase-1-Data-for-NBD1-Stabilizers-SION-719-and-SION-451-and-Advances-Both-Programs-in-Clinical-Development-for-Cystic-Fibrosis.html[28] Moleculin Reports Positive Topline Efficacy Results from U.S. Phase 1B/2 Clinical Trial Evaluating Annamycin for the Treatment of Soft Tissue Sarcoma Lung Metastases (MB-107). Retrieved June 5, 2025, from https://www.globenewswire.com/news-release/2025/06/04/3093575/0/en/Moleculin-Reports-Positive-Topline-Efficacy-Results-from-U-S-Phase-1B-2-Clinical-Trial-Evaluating-Annamycin-for-the-Treatment-of-Soft-Tissue-Sarcoma-Lung-Metastases-MB-107.html[29] REGENXBIO REPORTS NEW POSITIVE FUNCTIONAL DATA FROM PHASE I/II AFFINITY DUCHENNE® TRIAL OF RGX-202. Retrieved June 5, 2025 from https://ir.regenxbio.com/news-releases/news-release-details/regenxbio-reports-new-positive-functional-data-phase-iii[30] AbilityPharma’s IBRILATAZAR (ABTL0812) doubles overall survival in patients with squamous non-small cell lung cancer. Retrieved June 5, 2025 from https://abilitypharma.com/en/main-menu/media-center-2/press-release/abilitypharma%E2%80%99s-ibrilatazar-(abtl0812)-doubles-overall-survival-in-patients-with-squamous-non-small-cell-lung-cancer免责声明:药明康德内容团队专注介绍全球生物医药健康研究进展。本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。版权说明:欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「药明康德」微信公众号回复“转载”,获取转载须知。分享,点赞,在看,聚焦全球生物医药健康创新2
临床结果免疫疗法临床2期临床1期
100 项与 EBC-129 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 局部晚期恶性实体瘤 | 临床1期 | 美国 | 2023-04-28 | |
| 局部晚期恶性实体瘤 | 临床1期 | 新加坡 | 2023-04-28 | |
| 局部晚期恶性实体瘤 | 临床1期 | 中国台湾 | 2023-04-28 | |
| 胰腺导管腺癌 | 临床前 | 美国 | 2025-05-28 |
登录后查看更多信息
临床结果
临床结果
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用