更新于:2024-11-29
PRAME (Anocca)
更新于:2024-11-29
概要
基本信息
关联
100 项与 PRAME (Anocca) 相关的临床结果
登录后查看更多信息
100 项与 PRAME (Anocca) 相关的转化医学
登录后查看更多信息
100 项与 PRAME (Anocca) 相关的专利(医药)
登录后查看更多信息
2
项与 PRAME (Anocca) 相关的文献(医药)2020-01-01·Clinical & Translational Immunology
Ex vivo enrichment of PRAME antigen-specific T cells for adoptive immunotherapy using CD137 activation marker selection.
Article
作者: Hughes, Brendan ; Lee, Koon H ; Blyth, Emily ; Micklethwaite, Kenneth P ; Clancy, Leighton E ; Gowrishankar, Kavitha ; Singh, Mandeep ; McGuire, Helen M ; Street, Janine ; Luciani, Fabio ; Gottlieb, David J
OBJECTIVE:
Adoptive immunotherapy with ex vivo expanded tumor-specific T cells has potential as anticancer therapy. Preferentially expressed antigen in melanoma (PRAME) is an attractive target overexpressed in several cancers including melanoma and acute myeloid leukaemia (AML), with low expression in normal tissue outside the gonads. We developed a GMP-compliant manufacturing method for PRAME-specific T cells from healthy donors for adoptive immunotherapy.
METHODS:
Mononuclear cells were pulsed with PRAME 15-mer overlapping peptide mix. After 16 h, activated cells expressing CD137 were isolated with immunomagnetic beads and cocultured with irradiated CD137neg fraction in medium supplemented with interleukin (IL)-2, IL-7 and IL-15. Cultured T cells were restimulated with antigen-pulsed autologous cells after 10 days. Cellular phenotype and cytokine response following antigen re-exposure were assessed with flow cytometry, enzyme-linked immunospot (ELISPOT) and supernatant cytokine detection. Detailed phenotypic and functional analysis with mass cytometry and T-cell receptor (TCR) beta clonality studies were performed on selected cultures.
RESULTS:
PRAME-stimulated cultures (n = 10) had mean expansion of 2500-fold at day 18. Mean CD3+ percentage was 96% with CD4:CD8 ratio of 4:1. Re-exposure to PRAME peptide mixture showed enrichment of CD4 cells expressing interferon (IFN)-γ (mean: 12.2%) and TNF-α (mean: 19.7%). Central and effector memory cells were 23% and 72%, respectively, with 24% T cells expressing PD1. Mass cytometry showed predominance of Th1 phenotype (CXCR3+/CCR4neg/CCR6neg/Tbet+, mean: 73%) and cytokine production including IL-2, IL-4, IL-8, IL-13 and GM-CSF (2%, 6%, 8%, 4% and 11%, respectively).
CONCLUSION:
PRAME-specific T cells for adoptive immunotherapy were enriched from healthy donor mononuclear cells. The products were oligoclonal, exhibited Th1 phenotype and produced multiple cytokines.
2008-09-01·Leukemia1区 · 医学
Hematopoietic stem cells and progenitors of chronic myeloid leukemia express leukemia-associated antigens: implications for the graft-versus-leukemia effect and peptide vaccine-based immunotherapy
1区 · 医学
Article
作者: E M Sloand ; K Keyvanfar ; A J Barrett ; A S M Yong ; B N Savani ; R Eniafe ; J M Goldman ; K Rezvani
The cure of chronic myeloid leukemia (CML) patients following allogeneic stem cell transplantation (SCT) is attributed to graft-versus-leukemia (GVL) effects targeting alloantigens and/or leukemia-associated antigens (LAA) on leukemia cells. To assess the potential of LAA-peptide vaccines in eliminating leukemia in CML patients, we measured WT1, PR3, ELA2 and PRAME expression in CD34+ progenitor subpopulations in CML patients and compared them with minor histocompatibility antigens (mHAgs) HA1 and SMCY. All CD34+ subpopulations expressed similar levels of mHAgs irrespective of disease phase, suggesting that in the SCT setting, mHAgs are the best target for GVL. Furthermore, WT1 was consistently overexpressed in advanced phase (AdP) CML in all CD34+ subpopulations, and mature progenitors of chronic phase (CP) CML compared to healthy individuals. PRAME overexpression was limited to more mature AdP-CML progenitors only. Conversely, only CP-CML progenitors had PR3 overexpression, suggesting that PR1-peptide vaccines are only appropriate in CP-CML. Surface expression of WT1 protein in the most primitive hematopoietic stem cells in AdP-CML suggest that they could be targets for WT1 peptide-based vaccines, which in combination with PRAME, could additionally improve targeting differentiated progeny, and benefit patients responding suboptimally to tyrosine kinase inhibitors, or enhance GVL effects in SCT patients.
18
项与 PRAME (Anocca) 相关的新闻(医药)2024-05-21
Planegg/Martinsried, May 21, 2024. Medigene AG (Medigene or the “Company”, FSE: MDG1, Prime Standard), an immuno-oncology platform company focusing on the discovery and development of differentiated T cell immunotherapies for solid tumors and BioNTech SE (Nasdaq: BNTX, “BioNTech”) have announced that their collaboration to advance T cell receptor (TCR) immunotherapies against cancer will extend beyond the initial three-year term outlined at the signing of the agreement in February 2022. This extension will enable ongoing and future work required to potentially generate novel TCRs directed against multiple newly nominated antigen targets that could further expand the BioNTech warehouse of TCR candidates.
“We are pleased about the extension of our partnership with BioNTech aiming at the development of highly differentiated TCR-based therapies for patients suffering from solid tumors. The collaboration solidifies our position as a leader in the field of TCR generation while we continue to deliver very promising TCR candidates,” said Dr. Selwyn Ho, Chief Executive Officer at Medigene. “The ongoing work between our scientific teams reflects the strength of our partnership in the fight against cancer, and we look forward to making further progress in making a difference in the lives of cancer patients.”
In February 2022, Medigene and BioNTech entered into a global multi-target collaboration to discover and develop T cell receptor immunotherapies against multiple cancer targets including PRAME (PReferentially expressed Antigen in MElanoma). The agreement also included non-exclusive licenses to some of the proprietary technologies contained in Medigene’s End-to-End Platform including its PD1-41BB costimulatory switch protein.
About Medigene AG
Medigene AG (FSE: MDG1) is an immuno-oncology platform company dedicated to developing differentiated T cell therapies to effectively eliminate cancer. Its End-to-End Platform consists of multiple proprietary and exclusive technologies that generate optimal T cell receptors, armor and enhance these T cells to overcome the immunosuppressive tumor microenvironment (TME), and ensure the T cells drug product composition maximizes safety, efficacy and durability of response. This creates potential best-in-class, T cell receptor engineered T cell (TCR-T) therapies to treat multiple solid tumor indications for both its in-house product pipeline and partnering. Medigene’s lead TCR-T program MDG1015 is on track for IND filing in 3Q 2024 and CTA filing in 4Q 2024. For more information, please visit
About Medigene’s End-to-End Platform
Medigene’s immunotherapies help activate the patient’s own defense mechanisms by harnessing T cells in the battle against cancer. Medigene’s End-to-End Platform combines multiple exclusive and proprietary technologies to create best-in-class, differentiated TCR-T therapies. The platform includes multiple TCR generation and optimization technologies (e.g., Allogeneic-HLA (Allo-HLA) TCR Priming), as well as product enhancement technologies (e.g., PD1-41BB and CD40L-CD28 Costimulatory Switch Proteins, iM-TCR) to address challenges in developing effective, durable and safe TCR-T therapies. Partnerships with multiple companies including BioNTech and Regeneron, continue to validate the platform’s assets and technologies.
About Medigene’s PD1-41BB Costimulatory Switch Protein
Checkpoint inhibition via PD-1/PD-L1 pathway:
Cells of solid tumors are sensitive to killing by activated T cells but can escape this killing activity by producing inhibitory molecules known as ‘checkpoint proteins’, such as the Programmed Death Ligand 1 (PD-L1), on their surface. When this occurs, activated T cells which express PD-1, the natural receptor for PD-L1, are inactivated. The expression of PD-L1 is an adaptive immune resistance mechanism for tumors that can help them survive and grow.
The 4-1BB (CD137) costimulatory signaling pathway:
Effective T cell immune responses to antigens typically require both a primary antigenic stimulation via the T cell receptor (TCR) and costimulatory signals. The intracellular signaling domains of the 4-1BB protein offer a well-characterized pathway to costimulation and enhanced T cell responses.
Medigene’s PD1-41BB switch receptor turns the tumor’s attempted self-defense mechanism against the tumor by substituting the inhibitory signaling domain of PD-1 with the activating signaling domain of 4-1BB. Therefore, instead of inactivating T cells, the switch receptor delivers an activating signal to TCR-T cells. PD1-41BB-modified TCR-T cells proliferate strongly in the presence of PD-L1-positive tumor cells and kill more tumor cells upon repeated exposure. Additionally, switch receptor signals enable TCR-T cells to function better with low levels of glucose or high levels of TGFß, two conditions characteristic of strongly hostile tumor microenvironments.
About PRAME
PRAME (PReferentially expressed Antigen in MElanoma) is a tumor antigen of the cancer-testis-antigen family which is over-expressed in various solid and blood cancers. Expression in healthy tissue is limited to the testis, which itself is an immuno-privileged tissue that usually cannot be attacked by the body’s own immune cells. This renders PRAME very suitable as a target antigen for TCR-T therapies.
This press release contains forward-looking statements representing the opinion of Medigene as of the date of this release. The actual results achieved by Medigene may differ significantly from the forward-looking statements made herein. Medigene is not bound to update any of these forward-looking statements. Medigene® is a registered trademark of Medigene AG. This trademark may be owned or licensed in select locations only.
Medigene AG
Pamela Keck
Phone: +49 89 2000 3333 01
E-mail: investor@medigene.com
In case you no longer wish to receive any information about Medigene, please inform us by e-mail (investor@medigene.com). We will then delete your address from our distribution list.
引进/卖出免疫疗法细胞疗法
2024-05-08
·药明康德
▎药明康德内容团队编辑本期看点1. 治疗特应性皮炎的选择性肽疗法si-544的1b期临床试验结果积极,75%的患者的临床症状有客观改善。2. 下一代精准靶向疗法XPro使两名阿尔茨海默病患者的认知功能保持稳定超过3年。3. 美国FDA批准了在研先导编辑(prime editing)疗法PM359用于治疗慢性肉芽肿病(CGD)的IND申请,标志新一代基因编辑技术临床转化的重要里程碑。药明康德内容团队整理si-544:公布1b期临床试验数据selectION公司公布了其候选药物si-544在轻度至重度特应性皮炎患者中的1b期临床试验数据。si-544是一种选择性经优化的多肽疗法,可阻断离子通道Kv1.3。Kv1.3是一种参与效应记忆T(TEM)细胞激活和增殖的特殊离子通道。TEM细胞是许多自身免疫性疾病(如特应性皮炎、银屑病、类风湿性关节炎或多发性硬化)及某些罕见癌症(如淋巴瘤)的根源。在临床前研究中,si-544表现出了良好的疗效。该候选疗法通过在功能上抑制和消除疾病特异性、慢性激活的TEM细胞,同时保持完全的免疫能力,有望解决重大的未竟医疗需求。此次公布的结果显示,si-544的耐受性良好,没有观察到严重的不良反应、剂量限制毒性或安全信号。在接受si-544治疗的患者中,75%的患者的临床症状得到了客观改善,其中44%的患者在监测期结束时,皮肤变得光洁或接近光洁。用药后,这种改善的趋势维持了整个监测期。XPro:公布1b期临床试验中两名患者的数据INmune Bio公司公布了两名在完成1b期试验后继续接受XPro治疗超过3年的阿尔茨海默病患者的最新情况。XPro是一款旨在中和可溶性肿瘤坏死因子(TNF)的下一代精准靶向疗法。该候选疗法不会干扰促进正常功能和修复的TNF形式,这种特异性大大提高了有效性和安全性。两名阿尔茨海默病患者在完成为期3个月的开放标签试验和12个月的扩展试验后,在澳大利亚同情使用计划下继续接受XPro治疗超过三年。此次公布的结果显示,XPro长期给药的安全性和耐受性良好,这些患者已在持续的XPro治疗中保持稳定的认知功能超过3年。PM359:IND申请获得FDA许可Prime Medicine公司宣布,该公司为在研先导编辑疗法PM359递交的IND申请已经获得美国FDA的许可,用于治疗慢性肉芽肿病。这使该公司能够启动全球性的1/2期临床试验。新闻稿指出,这是先导编辑技术首个获得许可的IND,标着新一代基因编辑技术临床转化的重要里程碑。PM359是Prime Medicine在血液学和免疫学领域的首个候选疗法,针对的是慢性肉芽肿病的p47phox变体。PM359通过先导编辑器在体外修改自体造血干细胞(HSCs)。临床前研究显示,这些编辑器可以高效率纠正携带病因突变细胞的DNA。PM359已获得美国FDA授予的罕见儿科药物认定和孤儿药资格。二价诺如病毒候选疫苗:公布1期临床试验数据Vaxart公司公布了其口服二价诺如病毒候选疫苗片剂的1期临床试验的顶线结果。该候选疫苗的使用对象是处于哺乳期的母亲,旨在通过母乳将诺如病毒抗体从母体转移到婴儿,从而潜在地提高婴儿对诺如病毒感染的抵抗力。研究人员在这项研究中观察到哺乳期的母亲及其母乳中的抗体升高。在高剂量组母亲的母乳中,针对诺如病毒GI.1病毒株的抗体平均升高了4.0倍,针对GII.4病毒株的抗体平均升高6.0倍。安全性方面,没有出现与疫苗相关的严重不良事件,也没有出现剂量限制性药理毒性。LTI-03:公布1b期临床试验的初步数据Aileron Therapeutics公司公布了其用于治疗特发性肺纤维化(IPF)吸入性疗法LTI-03的1b期临床试验数据。LTI-03是一种新型的Caveolin-1相关多肽,由7个氨基酸组成,可抑制促纤维化信号传导并帮助关键上皮细胞存活。此次公布的结果显示,LTI-03低剂量组患者在接受治疗后,病理性基底样细胞和成纤维细胞中多种促纤维化蛋白的表达降低,与上皮健康相关生物标志物的表达增加。作为评估指标的8种生物标志物中,有7种表现出了积极趋势,其中3种具有统计学意义。安全性方面,低剂量LTI-03的耐受性良好,未观察到安全信号。LTI-03高剂量组的数据预计将于2024年第三季度公布。REM-422:两项1期临床试验完成首批患者给药Remix Therapeutics公司宣布,其潜在“first-in-class”的MYB mRNA降解剂REM-422的两项1期临床试验均已完成首批患者给药。MYB是多种实体瘤和血液恶性肿瘤的致癌驱动因子。REM-422是一种强效、选择性口服小分子mRNA降解剂,通过促进mRNA转录本掺入毒性外显子,引发无义介导的mRNA降解(NMD),可减少MYB mRNA及随后的蛋白表达,从而在MYB依赖性人类肿瘤模型中产生抗肿瘤活性。目前,REM-422正在一项针对复发或转移性腺样囊性癌(ACC)患者的1期临床试验和一项针对复发/难治性急性髓系白血病(AML)或高危骨髓增生异常综合征(MDS)患者的1期临床试验中接受评估。该公司还宣布,美国FDA授予了REM-422孤儿药资格,用于治疗ACC和AML。SNK01:IND申请获得FDA许可NKGen Biotech公司宣布,美国FDA已批准其在研自然杀伤(NK)细胞疗法SNK01用于治疗帕金森病的IND申请。SNK01是一款自体、非基因工程改造的NK细胞产品。它具有增强的细胞毒性和激活性受体的表达,有望成为治疗帕金森病的一种新方法。该公司预计将于2024年下半年启动针对帕金森病的1期临床试验。PRAME TCR/IL-15 NK(SY-307):IND申请获得FDA许可Replay公司和MD安德森癌症中心共同宣布,美国FDA已批准PRAME TCR/IL-15 NK(SY-307)的IND申请。PRAME TCR/IL-15 NK(SY-307)是一种由脐带血NK细胞开发而成的,用于治疗复发/难治性髓系恶性肿瘤的工程化T细胞受体(TCR)NK细胞疗法。这些细胞表达针对PRAME肿瘤相关新抗原的高亲和力TCR。PRAME具高免疫原性,在多种不同类型的癌症中都有表达,包括AML和MDS等血液系统恶性肿瘤,以及黑色素瘤、肉瘤、卵巢癌、子宫内膜癌、肺癌和乳腺癌等实体瘤。该候选疗法用于治疗复发/难治性AML和MDS患者的1/2期研究预计将于2024年第三季度启动。INNA-051:IND申请获得FDA许可ENA Respiratory公司宣布,美国FDA已批准其新型toll样受体2/6(TLR2/6)激动剂INNA-051干粉制剂的IND申请。INNA-051是一种不受病毒类型限制的鼻内抗病毒宿主防御免疫调节剂,是TLR2/6的强效潜在“first-in-class”激动剂。TLR2/6在识别病原体和触发先天性免疫反应中发挥着关键作用。此前,ENA Respiratory公司在流感模型中进行的2a期研究结果表明,INNA-051液体制剂能加速病毒清除并局部激发宿主的抗病毒防御能力。在此基础上,该公司开发了改进的干粉制剂,旨在为呼吸道病毒感染高危人群(包括老年人、患有基础疾病的人和有职业风险的人)提供一种方便的、每周仅需使用一次的鼻腔给药产品,以预防与呼吸道病毒感染相关的并发症。▲欲了解更多前沿技术在生物医药产业中的应用,请长按扫描上方二维码,即可访问“药明直播间”,观看相关话题的直播讨论与精彩回放参考资料(可上下滑动查看)[1] selectION Announces Positive Results from Phase 1b Clinical Trial Evaluating si-544 in Patients with Atopic Dermatitis. Retrieved April 30, 2024, from https://www.globenewswire.com/news-release/2024/04/29/2870968/0/en/selectION-Announces-Positive-Results-from-Phase-1b-Clinical-Trial-Evaluating-si-544-in-Patients-with-Atopic-Dermatitis.html[2] ENA Respiratory Announces FDA IND Clearance for its Prophylactic Intranasal INNA-051 - a First-in-Class Antiviral Innate Immunomodulator. Retrieved April 30, 2024, from https://www.globenewswire.com/news-release/2024/04/29/2870969/0/en/ENA-Respiratory-Announces-FDA-IND-Clearance-for-its-Prophylactic-Intranasal-INNA-051-a-First-in-Class-Antiviral-Innate-Immunomodulator.html[3] Prime Medicine Announces FDA Clearance of Investigational New Drug (IND) Application for PM359 for the Treatment of Chronic Granulomatous Disease (CGD). Retrieved April 30, 2024, from https://www.globenewswire.com/news-release/2024/04/29/2871079/0/en/Prime-Medicine-Announces-FDA-Clearance-of-Investigational-New-Drug-IND-Application-for-PM359-for-the-Treatment-of-Chronic-Granulomatous-Disease-CGD.html[4] Replay[5] Terns Pharmaceuticals Announces Data from Ongoing Phase 1 Pharmacokinetic Study of Allosteric BCR-ABL Inhibitor TERN-701 in Adult Healthy Volunteers and Highlights Potential for Competitive Differentiation. Retrieved April 30, 2024, from https://www.globenewswire.com/news-release/2024/04/29/2871639/0/en/Terns-Pharmaceuticals-Announces-Data-from-Ongoing-Phase-1-Pharmacokinetic-Study-of-Allosteric-BCR-ABL-Inhibitor-TERN-701-in-Adult-Healthy-Volunteers-and-Highlights-Potential-for-Co.html[6] Bitterroot Bio Announces Dosing of First Participants in a Phase 1, First-in-Human Study of BRB-002 in Healthy Volunteers. Retrieved April 30, 2024, from https://www.globenewswire.com/news-release/2024/04/29/2871160/0/en/Bitterroot-Bio-Announces-Dosing-of-First-Participants-in-a-Phase-1-First-in-Human-Study-of-BRB-002-in-Healthy-Volunteers.html[7] Replay and MD Anderson announce FDA clearance of IND application for first-in-class PRAME-targeted T-Cell Receptor Natural Killer (TCR-NK) cell therapy for hematological malignancies. Retrieved April 30, 2024, from https://www.globenewswire.com/news-release/2024/04/30/2872029/0/en/Replay-and-MD-Anderson-announce-FDA-clearance-of-IND-application-for-first-in-class-PRAME-targeted-T-Cell-Receptor-Natural-Killer-TCR-NK-cell-therapy-for-hematological-malignancies.html[8] Aileron Therapeutics Announces Positive Data from Cohort 1 of the Phase 1b Clinical Trial of LTI-03 in Idiopathic Pulmonary Fibrosis (IPF). Retrieved May 1, 2024, from https://www.globenewswire.com/news-release/2024/05/01/2873251/28652/en/Aileron-Therapeutics-Announces-Positive-Data-from-Cohort-1-of-the-Phase-1b-Clinical-Trial-of-LTI-03-in-Idiopathic-Pulmonary-Fibrosis-IPF.html[9] Context Therapeutics Announces FDA Clearance of IND Application for a Phase 1 Clinical Trial of CTIM-76. Retrieved May 6, 2024, from https://www.globenewswire.com/news-release/2024/05/02/2874098/0/en/Context-Therapeutics-Announces-FDA-Clearance-of-IND-Application-for-a-Phase-1-Clinical-Trial-of-CTIM-76.html[10] Ventus Therapeutics Announces First Participant Dosed in Clinical Study with an NLRP3 Inhibitor Licensed Exclusively to Novo Nordisk. Retrieved May 6, 2024, from https://www.businesswire.com/news/home/20240430835089/en[11] Arsenal Biosciences Announces First Patient Dosed in Phase 1/2 Clinical Trial of AB-2100 in Development as a Treatment for Clear-cell Renal Cell Carcinoma. Retrieved May 6, 2024, from https://www.businesswire.com/news/home/20240430131934/en/[12] Foresee Pharmaceuticals Announces First Subject Dosed in its First-in-Human Clinical Trial of FP-020. Retrieved May 6, 2024, from https://www.prnewswire.com/news-releases/foresee-pharmaceuticals-announces-first-subject-dosed-in-its-first-in-human-clinical-trial-of-fp-020-302131320.html[13] Aurion Biotech Announces Completion of Enrollment in Phase 1 / 2 Clinical Trial. Retrieved May 6, 2024, from https://www.businesswire.com/news/home/20240430312515/en[14] Alumis Announces First Participant Dosed in Phase 1 Clinical Trial of CNS Penetrant Allosteric TYK2 Inhibitor A-005. Retrieved May 6, 2024, from https://www.globenewswire.com/en/news-release/2024/04/30/2872215/0/en/Alumis-Announces-First-Participant-Dosed-in-Phase-1-Clinical-Trial-of-CNS-Penetrant-Allosteric-TYK2-Inhibitor-A-005.html[15] Debiopharm & Repare Therapeutics Announce First Patient Dosed in Phase 1/1b Mythic Trial Evaluating the Synthetic Lethal Combination of WEE1 AND PKMYT1 Inhibition. Retrieved May 6, 2024, from https://www.businesswire.com/news/home/20240430915822/en[16] IRLAB has Received Approval from the Swedish Medical Products Agency to Conduct a Phase I Study of the Drug Candidate IRL757. Retrieved May 6, 2024, from https://irlab.se/mfn_news/irlab-has-received-approval-from-the-swedish-medical-products-agency-to-conduct-a-phase-i-study-of-the-drug-candidate-irl757/[17] Remix Therapeutics Announces First Patients Dosed in Two Phase 1 Clinical Trials Investigating REM-422 for Treatment of Adenoid Cystic Carcinoma (ACC) and Acute Myeloid Leukemia/Myelodysplastic Syndromes (AML/MDS). Retrieved May 6, 2024, from https://www.prnewswire.com/news-releases/remix-therapeutics-announces-first-patients-dosed-in-two-phase-1-clinical-trials-investigating-rem-422-for-treatment-of-adenoid-cystic-carcinoma-acc-and-acute-myeloid-leukemiamyelodysplastic-syndromes-amlmds-302133787.html[18] INmune Bio Inc. Provides Update on Two Patients from the Phase 1b Alzheimer’s Disease Trial who Continue to Receive XPro™ Under Compassionate Use for Over Three Years. Retrieved May 6, 2024, from https://www.inmunebio.com/index.php/newsroom/2024-news/muneioncrovidespdateonwoatientsfromtheha20240430050503[19] Vaxart Announces Positive Results for Its Bivalent Norovirus Vaccine Candidate in Lactating Mothers. Retrieved May 6, 2024, from https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-positive-results-its-bivalent-norovirus-vaccine免责声明:药明康德内容团队专注介绍全球生物医药健康研究进展。本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。版权说明:本文来自药明康德内容团队,欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「药明康德」微信公众号回复“转载”,获取转载须知。分享,点赞,在看,聚焦全球生物医药健康创新
临床申请临床2期临床1期疫苗临床结果
2023-08-29
摘要患者对信使核糖核酸(mRNA)疫苗表现出良好的耐受性,编码分子的选择是灵活多样的。这些疫苗可以被设计成表达包含多个表位的全长抗原,而不受主要组织相容性复合体(MHC)的限制,相对容易控制,并且可以快速批量生产。美国食品药品监督管理局(FDA)于2021年批准了辉瑞和BioNTech生产的首个基于mRNA的新型冠状病毒病(COVID-19)疫苗,引发了mRNA疫苗研发热潮。基于上述特点和mRNA疫苗的发展,尤其是近五年来,mRNA肿瘤疫苗已成为研究热点,发展迅速。本文从抗原/靶点的选择和表达、载体和佐剂的应用、不同给药途径和临床前评价等方面对mRNA肿瘤疫苗的研究进展进行了分析,以反映mRNA肿瘤疫苗的发展趋势和面临的挑战。关键词:mRNA,癌症疫苗,肿瘤相关抗原,新抗原,mRNA递送载体,佐剂,给药途径。1.mRNA疫苗和mRNA癌症疫苗1961年,Brenner等人首次发现mRNA,它是基因作为蛋白质表达所必需的关键中间分子,含有与氨基酸(蛋白质的基本单位)相对应的密码子信息。1990年,Wolff等人首次证明,通过肌肉注射编码相应蛋白的纯RNA,可以在小鼠体内有效表达特定蛋白[如氯霉素乙酰转移酶和荧光素酶(Luc)];具体来说,βgLucβgAn RNA的蛋白表达以剂量依赖性和时间依赖性的方式发生;这项工作还提出了mRNA疫苗的概念。2020年,美国食品和药物管理局(FDA)紧急批准了Pfizer--BioNTech/BNT162b2和Moderna/mRNA-1273生产的两种基于mRNA的疫苗,用于预防2019年冠状病毒病(COVID - 19)。2021年,美国食品药品监督管理局(FDA)批准了Pfizer-BioNTech(上市名称为Comirnaty)生产的首个COVID-19疫苗,激发了mRNA疫苗的研发热情,并对mRNA癌症疫苗的突破产生了期待。迄今为止,研究人员已将mRNA用作疫苗平台[例如流感病毒、人类免疫缺陷病毒、冠状病毒、病毒抗原(狂犬病病毒糖蛋白、寨卡病毒和委内瑞拉马脑炎病毒的蛋白质),细菌病原体(结核分枝杆菌)和癌症]和蛋白质替代平台(例如factor IX、卵泡抑素、鸟氨酸经甲氨基甲酰基酶和促红细胞生成素),用于疾病的预防和治疗。mRNA疫苗具有许多共同特征。与质粒脱氧核糖核酸(DNA)和病毒载体存在因基因插入和/或感染而导致突变的风险不同,mRNA进入细胞质后可直接翻译成蛋白质;因此,mRNA疫苗是非整合的、非传染性的、耐受性良好的。mRNA也在细胞中短暂表达,允许重复接种。mRNA转录物中编码单位的选择是灵活多样的,允许编码抗原和免疫调节分子来诱导和调节适应性和先天免疫应答,并且含有多个表位的编码全长抗原可以由MHC Ⅰ类(MHC-Ⅰ)和Ⅱ类(MHC-Ⅱ)分子呈现,而不受MHC限制。体外转录(IVT) mRNA的生产不需要细胞,防止了蛋白质或病毒的污染,并允许快速、经济和容易的大规模生产。mRNA癌症疫苗利用编码肿瘤抗原或免疫调节分子的mRNA递送相应的蛋白,结合相关的递送载体和佐剂,诱导抗肿瘤应答。图1显示了mRNA癌症疫苗的发展时间表。抗肿瘤T细胞是介导这些疫苗治疗效果的主要预期效应细胞,图2总结了抗肿瘤T细胞产生和作用的机制。RNA在疫苗位点被DCs摄取,翻译并加工成抗原MHC Ⅰ/Ⅱ复合物并呈递到细胞表面。活化的DCs到达引流淋巴结,呈递的抗原MHC Ⅰ/Ⅱ复合物与淋巴结内分化簇8 (CD8)+/CD4+ T细胞(第一信号)表面的T细胞受体(TCR)结合,导致T细胞活化和增殖,共刺激信号分子[如CD80/CD86, OX40配体(OX40L)]与受体(如CD28, OX40)在T细胞(第二信号)和细胞因子[如干扰素(IFN) I,白细胞介素12 (IL-12), IL-1]与T细胞上的细胞因子受体结合(第三信号)。此外,CD4+ T细胞分泌的IL-2可促进CD8+ T细胞扩增。活化的T细胞在趋化因子[如CC -趋化因子受体7,CC -趋化因子配体(CCL) 5,CXC -趋化因子配体9/10]的作用下向肿瘤组织迁移并浸润,最大限度地发挥其分泌的效应器[如IFN-γ、肿瘤坏死因子(TNF)、穿孔素、颗粒酶]的抗肿瘤作用。内源性抗原主要通过MHC-Ⅰ分子呈递激活细胞毒性CD8+ T细胞,外源性抗原主要通过MHC-Ⅱ分子呈递激活辅助CD4+ T细胞。细胞毒CD8+ T细胞通常对靶细胞具有很强的直接杀伤作用,这些细胞是癌症疫苗预期诱导的主要效应细胞。2.mRNA癌症疫苗在临床前和临床研究中的进展疫苗的核心作用是提供能被人体免疫细胞识别的抗原,从而引发免疫反应。抗原/靶点的选择和表达、载体和佐剂的应用以及给药途径是疫苗设计中需要考虑的关键因素。表1总结了这些因素的进展在临床前和临床设置。表2总结了2016年至2021年期间使用基于mRNA的癌症疫苗的临床试验。2.1.选定抗原或靶标2.1.1.肿瘤相关抗原研制肿瘤疫苗的第一步是抗原的选择,抗原应具有较高的肿瘤特异性,并能诱导强而可控的抗肿瘤T细胞反应。肿瘤抗原根据其组织分布、表达水平和中枢耐受状态可分为TAAs和TSAs。TAAs在肿瘤中普遍过表达,在正常组织中也有表达,肿瘤特异性弱,中枢耐受性强,免疫原性弱,主要包括组织分化抗原和癌胚抗原。TAAs的中枢免疫耐受是利用这些抗原开发癌症疫苗的主要挑战。使用多个(如2-6个)共享TAAs的组合已成为临床开发靶向mRNA癌症疫苗的趋势。所选择的TAAs通常在相关肿瘤中广泛表达,当与不同的载体或佐剂结合时,可以诱导抗肿瘤免疫反应。2009年,Weide等人对鱼精蛋白(RNActive®)保护mRNA癌症疫苗进行了1/2期临床研究,采用GM-CSF作为佐剂,编码6种TAAs (Melan-A、酪氨酸酶、gp100、MAGE-A1、MAGE-A3和生存素)。这种疫苗是皮内注射。该疫苗显著降低了免疫抑制细胞(如外周血中的Foxp3+/CD4+调节性T细胞(Tregs)和骨髓抑制细胞),并增加了一部分患者的特异性T细胞。1例接受治疗的患者完全缓解,未发生大于Ⅱ级的不良反应(NCTO0204607)。2011年,Fotin-Mleczek等人对编码卵白蛋白(OVA)/PSMA/STEAP和PSMA的蛋白蛋白复合物mRNA癌症疫苗进行了临床前研究。结果表明,双组分mRNA肿瘤疫苗可通过TLR7诱导自佐剂作用,平衡适应性免疫反应,并具有持续的抗肿瘤作用(NCT00831467,NCT00923312)。2014年,Fotin-Mleczek 等人研究表明,蛋白复合OVA -编码mRNA的癌症疫苗与临床前放疗联合使用具有较强的协同抗肿瘤作用。编码4-5种前列腺特异性抗原[如CV9103 (NCT00831467)和CV9104 (NCT01817738)]或5-6种黑色素瘤和非小细胞肺癌TAAs[如CV9201 (NCT00923312)和CV9202 (NCT03164772)]的蛋白复合物肿瘤疫苗正在临床试验中。BN111是一种mRNA癌症候选疫苗,编码4种TAAs (NY-ESO-1、MAGE-A3、酪氨酸酶和TPTE)的固定组合,这些TAAs在黑色素瘤中普遍存在,并作为RNA -脂质复合物制剂(Lipo - MERIT)递送。BNT111单独或联合免疫检查点PD-1抑制剂可诱导不可切除黑色素瘤患者持续和强抗原特异性CD4+ / CD8+ T细胞反应和客观反应。超过5%的患者发生了相关不良事件,大多数不良反应为1-2级(NCT02410733)。基于这些结果,BNT111已获得FDA快速通道指定用于临床转化治疗晚期黑色素瘤(NCT04526899)。Lipo-MERIT mRNA癌症疫苗编码3个针对OC的TAAs (NCT04163094), 5个针对PC的TAAs[如BNT112 (NCT04382898)],或针对HNSCC和HNC的共享癌症抗原的固定组合[BNT113 (NCT04534205)],目前正在临床试验中。AML和骨髓瘤mRNA肿瘤疫苗的临床翻译也呈现出从单一TAA的应用[如WT1 (NCTO0834002、NCT00965224、NCTO1291420)]到多个TAA的联合应用[如WT1、PRAME、CMV pp65、癌睾丸抗原7、MAGE-A3 (NCTO1734304、NCT02405338、NCT01995708)]的趋势。研究发现,在43%的化疗后缓解的AML患者中,经WT1 mRNA电穿孔的DCs可预防或延迟复发(NCT00965224),改善的总生存率(OS)率或临床反应与诱导WT1特异性CD8+ T细胞反应(NCTO0965224, NCTO1291420)相关。TLR7 /8成熟DCs转染编码WT1、PRAME和CMV pp65的RNA可防止在完全缓解的AML患者亚群中复发(NCTO1734304)。mRNA肿瘤疫苗也在朝着个体化和精确化方向发展,初步趋势是从应用自体肿瘤[如AML (NCT00514189-已终止)、前列腺癌(NCT01197625)、NCT01278940、NCT008464562、NCT00961844-已终止]或肿瘤干细胞[如OC (NCT01334047-已终止)]衍生mRNA向采用个体化TAA 面板 (NCT01334047、NCT02709616、NCT02808364、NCT02808416)发展。负载完整肿瘤mRNA的DCs可以诱导针对肿瘤内广泛抗原的T细胞反应,甚至是患者独有的抗原(NCT01278940)。研究发现,用癌症干细胞衍生mRNA转染的树突状细胞可诱导患者产生免疫应答,并显示出有希望的初步安全性结果(NCT00846456)。含有3-13种不同TAA mRNA的个性化TAA面板脉冲DCs与良好的OS相关,并且治疗的患者没有出现Ⅲ/Ⅳ级不良事件(NCT02709616, NCT02808364, NCT02808416)。来源于自体肿瘤细胞或肿瘤干细胞的mRNA包含肿瘤细胞中的所有蛋白。这个策略简单可行,但其靶向性和有效性有待提高,同时也要考虑安全性。图1 mRNA癌症疫苗发展的时间轴。缩写:CART,电荷改变可释放转运体;CLAN,阳离子脂质辅助纳米颗粒;DCs,树突状细胞;DOTAP/DP7-C,1,2-二醇-3-三甲基丙烷氯/胆固醇改性阳离子肽DP7;LCP NPs,脂质/钙/磷酸(LCP)纳米颗粒;LPC,阳离子脂质体/鱼精蛋白复合物;LNPs,脂质纳米颗粒;Mann,甘露聚糖;PGCP NPs,聚(乳酸共乙醇酸)(PLGA)/G0-C14/神经酰胺聚(乙二醇)(PEG)(PGCP) NPs;PSA,前列腺特异性抗原;ssPAlmE-PALA,脂质纳米颗粒和a-螺旋阳离子肽“KALA”;triMN-LPR,阳离子脂质体(L)-一种阳离子聚合物(P)-mRNA (R),称为脂多聚体(LPR),通过A-D-甘露聚糖苷(triMN)的三天线功能化。2.1.2.肿瘤特异性抗原TSAs通常是由肿瘤细胞基因组中的非同义突变形成的肿瘤新抗原;这些抗原在正常细胞中不表达,具有很强的肿瘤特异性和免疫原性,中枢耐受性较弱。TSAs与抗肿瘤免疫反应之间的相关性已在多项研究中得到证实。对来自癌症基因组图谱的18个实体肿瘤数据中的数千个RNA序列的分析显示,每个肿瘤中新抗原的数量与T细胞细胞毒性活性相关基因的表达呈正相关。对来自癌症基因组图谱的515例患者的6个位点的RNA-seq数据分析表明,高水平的免疫原性突变表位与患者生存率的提高有关。具有高水平免疫原性突变的肿瘤具有更高水平的CD8A、PD-1和CTLA4。对619例结-直肠癌样本的全外显子序列分析表明,肿瘤中高水平的新抗原与肿瘤浸润淋巴细胞的增加和生存率的提高相关。在子宫内膜癌中也证实了新抗原水平与肿瘤浸润淋巴细胞数量之间的关系。此外,发现具有高水平新抗原的肿瘤比具有低水平新抗原的肿瘤具有明显的同质性。突变负荷大于每百万碱基10个体细胞突变的肿瘤(相当于表达基因中150个非同义突变)更容易形成免疫原性新抗原,而突变负荷小于每百万碱基1个体细胞突变的肿瘤不太可能形成免疫原性新抗原。大多数肿瘤的突变负荷为每百万碱基1-10个体细胞突变,通常可以形成T细胞识别的新抗原。Rajasagi等人利用全外显子测序和HLA -肽预测结合算法(即NetMHCpan)分析了13种不同肿瘤(2488个样本)的预测突变HLA-结合肽,结果表明每个肿瘤都可以产生数万到数千个新抗原,这表明新抗原在大多数肿瘤中是常见的。图2 mRNA癌症疫苗的作用机制图mRNA癌症疫苗的流行趋势是向个体化和精密度发展,旨在开发使用多种(20种)新抗原的mRNA癌症疫苗[如,IVAC MUTANOME (NCTO2035956)、IVAC_W_brel_uID和IVAC_W_brel_uID/IVAC_M_uID (NCT02316457)、RO7198457(NCT03289962、NCT04161755、NCT03815058、NCT04486378)、mRNA-4157 (NCT03313778、NCT03897881)、NCI4650/mRNA4650 (NCT03480152)、NCT03468244、NCT03908671]。2015年,Kreiter等人通过外显子组测序和MHC-Ⅱ表位预测结合算法分析了小鼠肿瘤细胞(如黑色素瘤细胞系B16F10、结肠癌细胞系CT26和乳腺癌细胞系4T1)中的突变肽,并制备了编码这些突变肽的RNA疫苗,在临床前评估它们的抗肿瘤作用。多新表位RNA在体内有效诱导T细胞应答,抑制小鼠肿瘤的生长和转移,大部分免疫原性突变体被CD4+ T细胞识别;即使RNA只编码一个新表位(如B16-M30),也能诱导更强的T细胞反应,并控制小鼠B16F10黑色素瘤的生长。Zhang等人对DOTAP/DP7-C脂质体进行了临床前研究,DOTAP/DP7-C脂质体作为载体和佐剂,装载了编码小鼠LLC细胞系LL2 (DOTAP/DP7-C/LL2)五种肿瘤新抗原的mRNA。DOTAP/DP7-C/LL2显著抑制原位和皮下LL2肿瘤的生长,刺激抗原特异性淋巴细胞反应。2017年,Sahin 等人发现基于RNA的多重新表位疫苗可诱导黑色素瘤患者的抗原特异性多克隆T细胞免疫反应;选择的新表位中有60%具有免疫原性,这些新表位诱导的主要T细胞反应是CD4+ T细胞反应,肿瘤转移明显减少,约75%的患者无进展生存期为27个月(NCT02035956)。2020年,Cafri等人发现编码20种新抗原的NCI4650/mRNA-4650可在胃肠道肿瘤患者中诱导新抗原特异性T细胞反应;选择的新抗原中有21%是免疫原性的,来自患者的新抗原特异性T细胞中有59%是CD4+ T细胞(NCT03480152)。虽然mRNA的制备是快速和经济的(良好生产规范级RNA可在3周内制备),但肿瘤新抗原的筛选和鉴定可能需要很长时间和昂贵,并且在疫苗制备过程中患者的病情可能发生变化,导致研究人员错过了患者的最佳治疗机会。基于高通量和生物信息学技术对基因深度测序和大数据集蛋白质组学分析的参数,治疗性肿瘤新抗原肽疫苗、RNA疫苗和融合DC -肿瘤细胞疫苗的制备时间分别约为160天、114天、103(89-160)天和10天。新抗原的筛选和鉴定速度直接影响mRNA新抗原疫苗的临床疗效,是mRNA新抗原疫苗面临的一大挑战。同时,肿瘤新抗原预测的准确性有待提高。表1 mRNA癌症疫苗在临床前和临床设置的概述2.1.3.免疫调节分子与肿瘤抑制基因编码CD70、CD40配体和组成活性TLR4的mRNA(命名为TriMix, NCT03788083);编码人OX40L、IL-23和IL-36γ的mRNA(命名为mRNA-2752, NCT02872025,NCTO3739931);和编码IL-12、IL-15、GM-CSF和IFN-α的mRNA(命名为SAR441000/BNT131、NCT03871348)是编码免疫调节分子的三种具有代表性的mRNA癌症疫苗,这类疫苗还包括编码TLR7/8激动剂和RIG-1激动剂的mRNA疫苗(CV8102/ RNA佐剂®、NCT03291002、NCT03203005);mRNA编码OX40L [mRNA-2416 (NCT03323398)];mRNA编码IL-12[MEDI1191 (NCT03946800), BNT151 (NCT04455620)];mRNA编码IL-12和IL-7 (BNT152, BNT153,NCT04710043);和编码BisCCL2/5i的mRNA。一些研究表明,编码免疫调节分子(如TriMix,mRNA-2752,BNT131和mRNA编码BisCCL2/5i)和肿瘤抑制基因(如PTEN或p53编码mRNA)的mRNA癌症疫苗作为单一疗法也具有抗肿瘤作用,通常与多种肿瘤抗原(如MAGE-A3、MAGE-C2、酪氨酸酶、gp100、生存素、hTERT和新抗原)和免疫检查点抑制剂(如抗PD-1、抗CTLA -4和抗PD - L1抗体)联合用作辅助治疗。2012年,Van Lint等人发现,结节内注射TriMix和TAA(如TRP2/WT1/P1A) mRNA可诱导DCs成熟并原位启动抗原特异性T细胞。与不加佐剂的荧光素酶(FLuc) mRNA脉冲DCs相比,TriMix显著降低了DCs中FLuc的表达,且LPS、单磷酰脂质A、聚(I:C)诱导的降低作用更强;而TriMix可以产生免疫刺激环境来改善T细胞反应,优于LPS。2016年Bialkowski等人发现HPV16-E7-TriMix mRNA可诱导CD8+ T淋巴细胞向肿瘤粘膜迁移,控制肿瘤生长。当与顺铂联合使用时,HPV16-E7-TriMix mRNA通过下调髓源性抑制细胞(MDSCs)和Tregs的数量来抵抗免疫抑制微环境,导致生殖道肿瘤完全消退。在多种小鼠肿瘤模型中,瘤内注射TriMix已被肿瘤浸润的树突状细胞吸收,然后呈交给肿瘤引流淋巴结的T细胞,诱导抗肿瘤T细胞反应和抗肿瘤作用。2013年和2016年,Wilgenhof等人发现,与TriMix共电孔的DCs和编码四种与HLA Ⅱ靶向信号(DCLAMP)(命名为TriMixDC-MEL)相关的黑色素瘤相关抗原(MAGE-A3、MAGE-C2、酪氨酸酶或gp100)之一的mRNA,在预处理的晚期黑色素瘤患者中具有良好的耐受性,并在两名患者(NCT01066390)中引起完全缓解和部分缓解。TriMixDC-MEL联合免疫检查点抑制剂(ipilimumab)在预先治疗的晚期黑色素瘤患者(NCTO1302496)中耐受并诱导高度持久的肿瘤反应。2020年,De Keersmaecker等人研究表明,在相当一部分晚期黑色素瘤患者中,TriMixDC-MEL和ipilimumab联合使用可诱导有效的CD8+ T细胞反应,并与患者的临床反应相关(NCTO1302496)。2019年Hewitt发现,在瘤内注射包裹在LNPs中的编码IL- 23、IL-36γ和OX40L的三联体mRNA,可以激活并招募多种免疫细胞(例如DCs和T细胞)进入肿瘤,从而诱导依赖于Batf3的交叉呈递DCs和细胞毒性CD8+ T细胞的持久抗肿瘤免疫。该疫苗与免疫检查点抑制剂(如抗PD -1、抗CTLA -4和抗PD - L1抗体)联合使用,在体内抗免疫检查点抑制剂模型中具有强大的抗肿瘤作用。2021年,Hotz等人发现BNT131联合抗PD -1抗体可显著提高荷瘤小鼠(如B16和MC38荷瘤小鼠)的生存率。2021年,Wang等人发现编码BisCCL2/5i LNPs的mRNA与编码PD-1配体抑制剂LNPs的mRNA联合可显著延长荷瘤(如原发性肝癌、结直肠癌和胰腺癌的肝转移)小鼠的生存期,且BisCCL2/5i可促进这些肿瘤对PD-1配体抑制剂的敏感性。表2 2016-2021年期间关于mRNA癌症疫苗的临床试验一些临床前研究表明,使用编码肿瘤抑制基因(如PTEN和p53)的mRNA治疗肿瘤是可行的。2018年,Islam等人研究发现,负载抑癌基因PTEN编码mRNA的聚脂包被多聚脂杂交NPs(如mRNA- pgcp NPs)可在体外和体内有效转染PTEN基因缺失的前列腺癌细胞,并通过抑制磷脂酰肌醇3-激酶Akt通路促进癌细胞凋亡,显著抑制肿瘤生长。2021年,Lin等人发现肿瘤抑制基因PTEN编码mRNA NPs可诱导PTEN突变的黑色素瘤细胞和PTEN缺失的前列腺癌细胞自噬和死亡。PTEN-mRNA NPs上调免疫抑制TME中的CD8+ T细胞和促炎细胞因子(如IL-12、TNF-α和IFN-γ),下调Tregs和MDSCs,并与抗PD -1抗体联合对这些肿瘤产生有效的抗肿瘤作用。2019年,Kong 等人研究发现肿瘤抑制基因p53-编码mRNA NPs可促进p53缺失型肝细胞和NSCLC细胞对哺乳动物雷帕霉素抑制剂靶点(依维莫司)的敏感性,且p53-mRNA NPs与依维莫司联用在体外和体内肝癌和NSCLC模型中具有显著的协同抗肿瘤作用。编码p53和肿瘤抗原(如生存素、hTERT、新抗原)的mRNA癌症疫苗目前正处于临床试验阶段(NCTO0978913、NCT02316457)。2.1.4. mRNA癌症疫苗与免疫检查点抑制剂的联合研究肿瘤发展过程中对TAAs的中枢免疫耐受和外周免疫耐受(如免疫检查点通路,TME)是癌症疫苗面临的两大挑战。两者都会影响癌症疫苗的效力和持续时间。为了靶向中枢免疫耐受,多个TAAs或多个TSAs的组合是mRNA癌症疫苗发展的主要趋势。为了靶向外周免疫耐受,将mRNA癌症疫苗与免疫检查点抑制剂(如抗PD -1、抗CTLA -4和抗PD - L1抗体)联合使用是mRNA癌症疫苗应用的另一个主要趋势。2018年,Liu等人对负载MUCI mRNA的LCP NPs联合抗-CTLA-4抗体治疗TNBC进行了临床前评估,结果表明LCP-mRNA NPs作为单一疗法或联合治疗的一部分(LCP-mRNA NPs + 抗-CTLA-4)可显著抑制肿瘤生长,且联合治疗的抑制效果明显强于LCP-mRNA NP单疗法。Wang等人研究表明,同时装载编码黑色素瘤相关抗原TRP2的mRNA和靶向PD-L1的小干扰RNA的LCP NPs可以在体外和体内有效地将mRNA传递到DCs中,并促进DCs的成熟。靶向PD-L1的小干扰RNA下调DCs中PD-L1的表达,增强抗肿瘤免疫和抗肿瘤作用,疫苗有效抑制肿瘤生长。2019年,Verbeke等人研究表明,单独使用Galsomes mRNA的体内抗肿瘤作用不明显,这种治疗可以增加免疫微环境中细胞毒性T淋巴细胞(CTL)、不变性NKT (iNKT)、NK和M1肿瘤相关巨噬细胞(TAMs)的数量;这些作者还发现,治疗对PD-1/PD-L1通路的负调控可能会限制其抗肿瘤作用。与单独接种疫苗相比,结合OVA mRNA Galsomes和抗PD-L1抗体显著增加脾脏iNKT细胞数量,降低脾脏DC细胞上PD-L1水平和脾脏增生iNKT细胞上PD-1水平,显著提高抗肿瘤效果。此外,Oberli等人表明,含有编码单一肿瘤抗原(例如gp100或TRP2)的mRNA的lnp和使用这两种抗原的序贯治疗在体内均具有显著的抗肿瘤作用;但两种方法的抗肿瘤作用无显著性差异。2.2.抗原或目标的表达2.2.1. mRNA分子的药效学用于制备疫苗的mRNA主要包括常规的非复制mRNA和病毒衍生的自扩增mRNA。mRNA的IVT是制备分子的主要技术,利用噬菌体RNA聚合酶(如T3、T7或SP6 RNA聚合酶)和含有目标抗原序列的线性化DNA模板。非复制性IVT mRNA的基本结构包括一个编码目标蛋白的开放阅读框(ORF)、一个位于5'和3'非翻译区(UTRs)两侧的7-methylgaunosine 5'帽和一个3' poly(A) 尾。5'帽和3'poly(A)可以在IVT过程中添加,也可以在初始IVT后酶促添加。自我扩增的mRNA包含两个ORFs,一个编码目标抗原序列,另一个编码病毒复制机制,从而实现持久的细胞内RNA扩增。一种由编码CEA的甲病毒复制子组成的mRNA疫苗(称为AVX701)正在临床试验中(NCTO0529984, NCTO1890213)。与蛋白质或肽疫苗不同,mRNA癌症疫苗产生效果的第一步是编码蛋白的序列信息可以翻译成功能蛋白。影响翻译过程的因素包括正调节因素、负调节因素和双向调节因素。正调控因子总结如下:①5′帽及其修饰物[如抗反向帽类似物CleanCap]可招募真核翻译起始因子4E,促进核糖体识别和翻译起始,消除mRNA序列中的游离磷酸基团,显著增强mRNA的稳定性。②poly(A)序列及其修饰(如长度’)可以减缓RNA外切酶的降解过程,从而增加稳定性,延长体内半衰期,提高mRNA的翻译效率。③UTR优化[例如,由a-珠蛋白和β-珠蛋白衍生的3' UTR序列,AU和GU-富集序列;3' UTR中的稳定元素;5' UTR中的GCC-(A/G)- CCAUGG,短而松散的5' UTR135]和ORF的密码子优化(如尿苷的缺失,G:C含量的富集,同义频繁密码子,具有较高转移RNA丰度的密码子)可以提高mRNA的稳定性和蛋白质翻译。表达载体中的表位通过不同的序列和信号肽(例如,内核/溶酶体信号分选片段和跨膜-细胞质结构域)连接,以增加IVT,提高抗原在细胞内加工和呈递的靶向性。④核苷修饰[如假尿嘧啶(Ψ)、1-甲基假尿嘧啶、5-甲基胞苷(5meC)和n -乙酰胞苷修饰转录后RNA]和纯化IVT-mRNA(如Mg2+31、温度、高压液相色谱和快速蛋白液相色谱)以减少双链RNA的污染,可以降低分子的先天免疫激活,增加蛋白质翻译。与未修饰的mRNA相比,核苷修饰的mRNA (5meC, Ψ)可显著促进小鼠FLuc的表达。LCP(修饰mRNA)的体内抗肿瘤作用明显强于LCP(未修饰mRNA)。负调控因素包括:①细胞外RNases酶能快速降解裸mRNA。②IVT产生的双链RNA杂质可以结合细胞质中的模式识别受体(PRRs)[如RIG - Ⅰ,黑色素瘤分化相关蛋白5 (一种RIG -Ⅰ受体),蛋白激酶RNA激活(也称为真核翻译起始因子2 alpha激酶2),2'-5'-寡腺苷酸合成酶]和内体(如TLR3)来激活特定途径[如RIG -Ⅰ /MAD5→线粒体抗病毒信号蛋白→IFN Ⅰ,(IFN Ⅰ→)蛋白激酶RNA激活→真核翻译起始因子2alpha, (IFN Ⅰ→)2′5′-寡聚腺苷酸合成酶→核糖核酸酶L, TLR3→含有Toll/IL-1受体结构域的适配器诱导IFN-β→IFN Ⅰ],可抑制mRNA翻译,促进mRNA酶解。双向调节因子包括未修饰的单链RNA作为病原体相关分子模式(PAMP),它可以结合内体中的PRRs[如TLR7, TLR8]来激活特定途径(TLR8→髓样分化因子88→促炎细胞因子;TLR7→骨髓分化因子88→干扰素调节因子7→IFN Ⅰ)。一方面,mRNA可以激活先天免疫反应(DC成熟和激活),进而激活适应性免疫反应(T和B细胞免疫反应);另一方面,过早和过度激活IFN Ⅰ可抑制mRNA翻译,促进mRNA酶解,促进DC和T细胞凋亡。过度强烈的炎症反应也会引起毒副作用。在T细胞中,Ⅰ型IFN受体信号的激活先于TCR信号的激活,可作为促进免疫应答的真正第三信号。Udhayakumar等人表明,与含有未修饰mRNA的RALA mRNA纳米复合物相比,含有Ψ-和 5meC-修饰mRNA的mRNA纳米复合物诱导了有效的抗原特异性细胞毒性T细胞反应,并且具有优越的疗效,修饰的(5meC,Ψ) mRNA纳米复合物通过抑制IFN-β活化显著降低了Ⅰ型IFNs对CTLs的抑制作用,并有效诱导了CTLs。相比之下,Oberli等人表明,未修饰mRNA LNP疫苗在外周血中诱导的CD8 T细胞应答(7.8%)比核苷修饰mRNA (5meC,Ψ) LNP疫苗(1.0%)强得多,并表明Ⅰ型干扰素是保护性CD8 T细胞应答所必需的。这些相互矛盾的结果可能与双向调节因素有关。2.2.2.疫苗抗原表达方法根据抗原的表达,疫苗可分为多肽或蛋白质疫苗、细胞疫苗(如肿瘤细胞疫苗、DC疫苗和工程细胞疫苗)、核酸疫苗(如DNA和RNA疫苗)和病毒载体疫苗。肽或蛋白疫苗是广泛使用的疫苗类型。抗原肽的序列定义明确,易于控制。肽疫苗包括短肽疫苗和长肽疫苗。短肽疫苗有一些缺点,包括蛋白质水解导致抗原降解和免疫反应持续时间较短,短肽可以结合许多核细胞表面的MHC Ⅰ分子,这些核细胞作为非专业抗原呈递细胞APCs),通常不含共刺激信号,导致抗原耐受和T细胞功能障碍。长肽疫苗通常含有20-30个氨基酸,可激活CD4+和CD8+ T细胞。蛋白质疫苗也能诱导T细胞反应;然而,长肽通常比蛋白质更有效地被APCs内化和加工。长肽疫苗的缺点包括对酶降解的敏感性、快速清除和注射部位吸收不足。细胞疫苗主要包括癌细胞疫苗和DC疫苗。癌细胞疫苗是用自体或异体灭活的全细胞及其衍生物(如细胞裂解物、DC融合衍生物、表达TSAs或免疫增强因子的修饰全细胞和肿瘤衍生mRNAs),它们通常包含细胞的所有抗原,在疫苗设计和生产之前不需要费力地鉴定,从而导致相对快速的制备和某些个性化特征。然而,使用癌细胞的方法不能准确地测定和控制相应的肿瘤抗原,质量控制困难,而且癌细胞往往含有较少的特异性抗原,导致免疫原性较弱和潜在的致癌性。DCs是最有效的APCs,在连接先天和适应性免疫反应中起着核心作用。DC疫苗一般使用自体DC作为载体来表达和呈递抗原。1996年,Boczkowski等人证明了使用肿瘤来源的mRNA癌细胞脉冲DCs的可行性。2017年之前,在大约24项临床试验中,mRNA癌症疫苗使用DCs作为载体。然而,DC疫苗的制备工艺复杂,生产昂贵,质量控制困难。此外,患者必须具有相对正常的免疫功能,没有化疗或其他治疗引起的骨髓抑制,并提供大量功能性DCs,导致可用疫苗数量有限。核酸疫苗是用编码抗原的核酸(如DNA、RNA)制备的。mRNA疫苗的特点已在本文第1部分进行了描述。与mRNA相比,DNA必须进入细胞核才能翻译成相应的抗原,这有插入突变引起的潜在风险,可能比mRNA更不安全。总的来说,核酸对降解敏感,不稳定,半衰期短,导致裸核酸被APCs吸收的效率较低。新的载体和给药途径。用于提高核酸的吸收和呈递效率(下文讨论)。有限的研究表明mRNA癌症疫苗与肽或蛋白癌症疫苗相比具有抗肿瘤优势。用体外合成的鸡OVA RNA脉冲的DCs比用OVA肽脉冲的DCs在体外刺激OVA特异性的初级CTL反应更有效。在原位治疗性肿瘤模型中,DOTAP/DP-C/mRNA编码5种新抗原在诱导脾脏产生活化T细胞(CD3+ CD8+ IFN-γ+)方面明显强于DOTAP/DP-C/突变肽。LCP(修饰mRNA)的体内抗肿瘤作用显著强于LCP (TRP2肽/CpG)。编码IL-23、IL-36和OX40L的三联体mRNAs提高MC38-S荷瘤小鼠存活率的能力明显强于相应的蛋白处理。肽抗原通常只含有一个表位,而mRNAs编码的全长抗原含有多个表位,可以诱导T细胞靶向这些表位,产生更强的抗肿瘤作用。病毒载体疫苗是利用病毒作为表达或呈递抗原的载体制备的。目前,被广泛研究的病毒载体包括痘病毒、腺病毒和疱疹病毒。出于安全考虑,采用复制缺陷病毒或减毒病毒。痘病毒可包含多个基因,复制和转录仅限于细胞质,插入突变的风险较低,表达产物可呈现MHC Ⅰ和Ⅱ。非禽类痘病毒可以诱导宿主产生免疫反应,从而使其只能使用一种或最多两种疫苗。重组鸟痘病毒可以多次接种,其病毒外壳蛋白不能在哺乳动物细胞中产生,并且病毒不能诱导宿主产生免疫反应来中和病毒。重组腺病毒载体易于设计,作为疫苗和基因治疗药物的载体已显示出实用性;然而,它们的免疫原性会影响疫苗的效果。疱疹病毒有广泛的宿主范围;可感染神经细胞、外周血单核细胞和DCs;并且具有复制周期短、容量大、安全性相对较好的特点。此外,其他载体,如细菌和酵母,在临床前研究中显示出作为疫苗载体的潜力。总的来说,免疫原性、致癌性、传染性、包装能力有限和病毒载体生产困难是广泛应用的挑战。2.3.mRNA癌症疫苗的载体开发具有良好的安全性、靶向性、稳定性、自佐剂效应、负载能力和通用性的mRNA递送载体,能够高效、持续地递送和呈递抗原,激活APCs,是mRNA肿瘤疫苗领域的一个基本方向。mRNA肿瘤疫苗在临床前采用的载体如表3所示。mRNA癌症疫苗中使用的主要载体之一是脂质体及其衍生物。1995年,Conry等人显示了编码人CEA复合物的脂质体mRNA的体液免疫原性,首次在临床前研究中证实了mRNA癌症疫苗的概念验证。甘露糖可以与DCs表面表达的甘露糖受体结合,利用靶向DCs的载体促进mRNA的高效传递和转染。2011年,Perche等人研究表明,携带mRNA的Man11-LPR100转染DCs的效率是无糖LPR100的4倍,并且在体内具有更好的抗肿瘤作用。由于甘露糖与其受体之间的结合力较弱,增加载体表面甘露糖的密度可能是提高甘露糖修饰LPR递送效率的有效途径。2018年,Le Moignic 等人研究表明,与单甘油三酯- LPR相比,三甘油三酯- LPR可以更有效地诱导抗原转染,通过在注射部位诱导局部炎症反应,将更多的DCs招募到引流淋巴结,并更有效地诱导抗原特异性免疫反应。2018年,Wang 等人利用甘糖醇偶联物(MPn-CHs)制备了DCs靶向脂质体(MPn-LPs)作为mRNA载体,结果表明MP1000-LPs负载mRNA (MP1000-LPX)具有良好的转染效率,MP1000-LPX主要通过增强DCs上甘糖受体(如CD206)的表达来增强mRNA的表达。2020年,Son等人研究表明,采用多糖包被二氧化硅纳米颗粒制备的Mann -capsules可以通过Dectin-2或TLR-4激活骨髓源性树突状细胞(BMDC),且Mann -capsules促进BMDC分化和成熟的能力明显强于PEI或脂质体;此外,PEI和脂质体具有高毒性。LNPs似乎是一种很有前途的mRNA癌症疫苗载体。LNPs的成分主要包括可电离脂质,促进mRNA的自组装和内体释放;磷脂,支持脂质双层结构;胆固醇,一种稳定剂;脂质锚定聚乙二醇,延长配方的半衰期。还考虑了筛选和鉴定这些媒介的高通量技术。2017年,Oberli 等人构建并优化了LNP库,发现含有编码肿瘤抗原的mRNAs(如gp100和TRP2)与LNPs作为佐剂结合,可有效诱导抗原特异性CD8+ T细胞,抑制肿瘤生长,延长小鼠OS。2018年,McKinlay等人对基于两亲性CARTs的mRNA载体库进行了高通量筛选研究,结果表明,与单一CART或脂质体 2000相比,双CART可将体外淋巴细胞mRNA转染效率提高9倍;体外mRNA转染效率为80%,小鼠淋巴细胞mRNA转染效率为1.5%。2019年,Miao等人建立了一种高通量的可电离类脂质构建技术,可在一天内合成数千种脂质制剂,并使用DCs(如HeLa细胞、BMDCs或骨髓源性巨噬细胞)高通量评估LNPs的转染效率。结果表明,mRNA LNPs通过干扰素基因通路的胞内刺激物诱导APC成熟,增强抗肿瘤作用。2021年,Meng等人发现含有CpG核心的VLVPs可以促进DC成熟和抗原呈递、抗原特异性CD8+ T细胞在淋巴器官中的增殖和肿瘤中的T细胞浸润,并减少免疫抑制细胞(如肿瘤相关骨髓源性抑制细胞和表达精氨酸酶1的抑制性DCs)。以LNPs为载体的mRNA癌症疫苗[如编码新抗原的mRNA-4157 (NCTO3313778、NCTO3897881)和编码突变蛋白的V941 (NCT03948763)]目前正处于临床试验阶段。多阳离子肽鱼精蛋白和DCs是mRNA癌症疫苗中采用的另外两种主要载体。鱼精蛋白和DCs的特点及其作为mRNA癌症疫苗载体的研究进展分别见第2.1.1节和2.2.2节。鱼精蛋白可以保护mRNA不被血清RNA酶降解,促进mRNA的传递。此外,鱼精蛋白可与脂质体联合使用(例如,阳离子脂质体-鱼精蛋白,LPC和VLVP)。2000年,Hoerr等人发现脂质体包裹的浓缩RNA-肽复合物可诱导抗原特异性细胞免疫反应和体液免疫反应,裸RNA和蛋白蛋白保护的RNA在体内均可诱导特异性免疫反应,而受保护的RNA在体外稳定时间较长。Mai等人的研究表明,携带细胞角蛋白19编码mRNA的LPC经鼻递送可诱导小鼠APC成熟和强烈的细胞免疫反应,并降低肿瘤的生长。载体对疫苗的效力有关键影响。Phua等人表明,在没有NP载体的情况下,鼻内接种裸mRNA不能诱导抗肿瘤免疫反应。然而,临床前和临床研究都在综合考虑所选靶点、佐剂和递送方法的基础上证明了裸mRNA的可行性和有效性。发现FLT3可增强结内裸RNA的抗肿瘤作用。结内HPV16-E7-TriMix裸mRNA和瘤内TriMix裸mRNA均可诱导有效的抗肿瘤T细胞反应。结节内裸mRNA多重新表位疫苗诱导有效的抗原特异性T细胞免疫应答(NCT02035956)。2.4.佐剂在mRNA癌症疫苗的临床前研究中开发的一类主要佐剂[如LPS73, poly(I:C), Td, CpG]是PAMPs,它通过PAMP-PRR途径激活DCs,然后调节先天和适应性免疫反应。作为一种强效的回忆抗原,Td可通过CCL3106促进DC迁移,提高抗肿瘤作用。LPS辅助处理进一步提高了CD8+ T细胞水平和LNP mRNA诱导细胞的抗肿瘤活性。mRNA- CART联合CpG的体内抗肿瘤作用明显强于mRNA-CART和裸mRNA与CpG的联合,也强于mRNA-CART与TLR7配体或CD80 /86 mRNA的联合。大多数新型载体(如脂质样材料C1、DOTAP/ DP7-C、Mann-capsule、LNPs)兼具载体和佐剂的特性,其中一些载体具有佐剂的功能,类似于PAMPs。Mann -capsule通过Dectin-2或TLR-4激活BMDCs,葡聚糖-capsule通过CD206、CD209或巨噬细胞诱导的C型凝集素激活BMDCs。DP7-C、DOTAP和mRNA分别通过TLR2、TLR4和TLR7激活DCs。DOTAP/DP7C诱导DC成熟和抗原呈递的能力明显强于DOTAP、poly(I:C)和CpG。Cl或C1 mRNA可通过TLR4依赖的核因子κB信号通路促进BMDC活化,C1- OVA mRNA的体内抗肿瘤作用是TLR4依赖的CpG,因为VLVP中含有的佐剂,可以提高疫苗的效果,阻止PD-1在T细胞中的表达。然而,这些类PAMP佐剂可能影响mRNAs的翻译效率和降解,并具有潜在的毒性,如2.2.1节所述。开发既不影响mRNA翻译效率,又能积极调节多种先天和适应性免疫反应,促炎作用相对温和,毒性较低的新型佐剂已成为重要方向。TAA mRNA的免疫原性可以通过GM-CSF mRNA的共递送而增强。FLT3配体可促进浆细胞样DCs、经典DCs和NK细胞的扩增;诱导T辅助1型微环境;增强肿瘤内T细胞浸润及裸RNA的抗肿瘤作用。mRNA LNPs可通过刺激干扰素基因依赖性激活Ⅰ型IFN,诱导APC成熟,限制全身细胞因子表达,增强抗肿瘤作用。用于mRNA Galsomes的佐剂α-GC可通过DCs呈递激活iNKT细胞。活化的iNKT细胞可与DCs发生双向正调节作用,可正向调节NK细胞和免疫抑制细胞(如MDSCs和M1 TAMs),不影响mRNA翻译效率,促进直接和间接的抗肿瘤作用。编码免疫共刺激分子的mRNA可用作佐剂(见2.1.3节)。TriMix (NCT01066390, NCTO1302496);mRNA-2752 (NCT02872025);mRNA编码人OX40L (mRNA-2416, NCT03323398);编码IL-12、IL-15、GM-CSF和IFN-α的mRNA (BNT131,NCTO3871348);编码IL-12的mRNA (MEDI1191,NCT03946800);和mRNA编码的TLR7 /8激动剂和RIG-1-兴奋剂(RNA佐剂®),NCT03291002,NCT03203005]正在临床试验中作为辅助治疗与免疫检查点抑制剂联合使用。表3 在临床前环境中用于mRNA癌症疫苗的递送系统概述2.5.给药途径给药途径直接影响疫苗的效力,mRNA癌症疫苗通常采用的给药途径包括静脉注射、皮内、皮下、肌肉注射、结内和肿瘤内给药。静脉注射允许更大的疫苗量和直接将疫苗输送到淋巴器官,但也有更大的系统性毒性风险。皮下注射疫苗的真皮含有APCs(如,DCs和巨噬细胞)以及血管和淋巴血管,但主要由致密结缔组织组成,导致通过皮内途径给药时疫苗量很小。皮内给药也可导致注射部位的不良反应(例如,肿胀、疼痛、红斑和瘙痒)。皮下注射疫苗的皮下区域含有比真皮更少的APCs;然而,它主要由脂肪组织的松散网络组成,允许通过皮下途径注射更大的注射量,从而引起更少的局部副作用(如疼痛)。肌肉注射疫苗的肌肉含有致密的血液网络,可以帮助招募和再循环不同类型的免疫细胞(如,浸润性APCs)到注射部位。肌内给药比皮内给药的注射量大,局部副作用也比皮内和皮下给药轻。结内注射疫苗的淋巴结含有多个APCs,结内给药具有较高的递送效率,允许小剂量的疫苗,但涉及复杂的程瘤内给药主要用于编码免疫共刺激分子的mRNA疫苗(例如TriMix, CV8102, mRNA-2752, mRNA-2416, BNT131和MEDI1191)作为免疫辅助治疗,也允许小剂量的疫苗,涉及复杂的程序。准确预测特定疫苗的最佳给药途径是困难的,建议通过直接比较研究来选择疫苗的最佳给药途径。结内注射的Luc-RNA在体内的转染效率优于皮内或皮下注射。经静脉注射OVA RNA复合DOTAP-DOPE诱导的CTLs杀伤作用优于皮内或皮下注射(静脉注射>皮内注射>皮下注射)。结内递送OVA mRNA联合TriMix诱导的抗原特异性T细胞的体内细胞毒性明显强于皮内递送疫苗。皮内接种E7 mRNA单糖化-LPR诱导的抗原特异性T细胞应答明显强于皮下接种。通过CART有效地将mRNA传递到APCs中(次要淋巴细胞APCs优先通过静脉注射靶向,而局部APCs通过皮下注射靶向)。肿瘤内三次给药的编码IL-23、IL-36和OX40L的三联体mRNAs的抗肿瘤作用显著优于同种药物的皮内或皮下给药;然而,在同一剂量下,通过这些途径的抗肿瘤效果没有显著差异,这表明对肿瘤疫苗的给药效果也与给药频率有关。鼻黏膜富含APCs和免疫细胞,临床前研究表明经鼻内注射的mRNA癌疫苗初步有效。鼻内接种mRNA纳米颗粒和鼻内递送LPC mRNA均可诱导抗肿瘤免疫应答。3.mRNA癌症疫苗的挑战和趋势迄今为止,已有数百种癌症疫苗进行了临床评估,美国食品和药物管理局(FDA)批准了三种治疗性癌症疫苗[卡介苗 (TheraCys®),一种牛分支杆菌减毒活株,用于治疗非肌肉浸润性膀胱癌;Sipuleucel-T (Provenge®),一种用于治疗转移性去势抵抗性前列腺癌的DC疫苗;以及用于治疗晚期黑色素瘤的溶瘤性疱疹病毒疫苗(talimogene laherparepvec, T-VEC) (Imlygic®)和两种预防性癌症疫苗(HPV疫苗和乙型肝炎病毒疫苗)。影响mRNA肿瘤疫苗研制的因素主要包括分子本身的内在因素和外部因素[如对肿瘤抗原的中枢耐受性(见第2.1.1节)、肿瘤和HLA的异质性以及肿瘤免疫微环境]。这些因素深刻地影响了mRNA癌症疫苗的发展。从内在因素来看,研究人员通过改进mRNA的结构和序列,改进mRNA的制备和纯化技术(见第2.2.1节),开发新的传递载体(见第2.3节),在一定程度上提高了mRNA癌症疫苗的有效性。肿瘤异质性的本质是肿瘤细胞的基因组异质性,导致抗原异质性,这是影响抗肿瘤T细胞反应产生的关键因素,也是开发个性化癌症疫苗的主要原因。HLA异质性主要是指个体间HLA分子类型的不同,导致个体间这些分子对肿瘤抗原的结合区域或结合亲和力存在差异,从而影响抗肿瘤T细胞反应的产生和强度。HLA异质性是由HLA等位基因在不同民族和地区人群中的多态性引起的。HLA Ⅰ等位基因包括9-11个常见超型,其覆盖率为90%。HLA-A loci (HLA-A等位基因:A*0101、A*0201、A*0301、A*1101和A*2402)占HLA-Ⅰ等位基因的60%,HLA-B loci (HLA-B等位基因:B*0702、B*0801、B*2705、B*3501和B*5701)占这些等位基因的35%以上。TME由免疫细胞、间充质细胞以及多种细胞因子和组织因子组成,在肿瘤发生和免疫逃逸中起重要作用。大质量内的间质压力可减少大分子(如抗体)和效应细胞(如T细胞)的扩散。大多数实体瘤也缺乏T细胞共刺激分子。TME通常含有免疫抑制细胞,包括CD4+Tregs, MDSCs,抑制性CD8+T细胞,M2 TAMs和调节性NK/NKT细胞。这些免疫抑制细胞和TME中的肿瘤细胞可以释放大量可溶性的免疫抑制因子,包括转化生长因子β、IL-10、PD-L1、吲哚胺2,3-双加氧酶和血管内皮生长因子等,进入微环境。考虑到肿瘤和HLA的异质性,肿瘤新抗原疫苗的开发在理论上比TAA定向疫苗具有更强的特异性抗肿瘤作用和更弱的毒副作用,一直是癌症疫苗研究的主要热点(见2.1.2节)。鉴于肿瘤免疫微环境,开发基于免疫的联合疗法(例如,与佐剂或免疫检查点抑制剂联合)已成为癌症疫苗应用的一个关键趋势(见第2.1.4和2.4节)。4.讨论产品的临床前评价是进入临床转化的前提,合理的评价可以提高预测临床结果的可靠性。临床前评价所采用的参数、技术和方法是疫苗质量标准的重要组成部分。疫苗的临床前评价应充分阐明疫苗的作用和机制,而mRNA肿瘤疫苗临床前评价的重点是确定抗原特异性T细胞反应的产生(如T细胞的数量和活化)和效果(如T细胞的杀伤作用和抗原亲和力)。一般来说,在临床前评估中,定量的体外和体内试验用于评估mRNA癌症疫苗的效果和机制。根据目前对抗原特异性T细胞免疫应答的认识,评价参数主要包括①鉴定疫苗的理化特性;②mRNA转染APC的效率(如结合与摄取、内化与转运、表达与分布);③APCs的分化、成熟和抗原提呈;④疫苗免疫刺激;⑤细胞免疫原性(如CTL的产生、增殖和靶细胞杀伤);⑥体液免疫原性;⑦抗肿瘤作用及相关机制;⑧初步毒性(如细胞毒性、内脏毒性和溶血)。诸如颗粒大小、电荷、对mRNA的结合能力和负载率、稳定性(如时间、温度和血清稳定性)、聚乙二醇化和硬度等因素可影响抗原递送(如淋巴引流)。Son等人研究表明,尺寸为~220 nm的Mann-capsules具有良好的变形能力,通过50 nm孔膜后的回复率约为30%。与基于蛋白质或肽的疫苗不同,mRNA疫苗开发的第一步是确保mRNA序列中编码的信息能够有效地翻译成相应的蛋白质或肽。mRNA的体外和体内转染效率是mRNA疫苗临床前评价的重要参数,提高mRNA的转染效率是提高mRNA疫苗药效学的关键途径。然而,APCs的体外转染效率可能存在差异,DOTAP/ DP7 - 增加了绿色荧光蛋白mRNA在APCs(包括293T、JAWSII、DC2.4和BMDCs)中的体外转染效率分别为84.87±3.21%、12.23±1.35%、28.49±2.46%和14.51±2.35%。为了验证mRNA在体外和体内的转染效率,我们以多种APCs作为体外转染模型,在体内检测不同器官的多种APCs的mRNA转染效率。增强绿色荧光蛋白(FLuc)是实验中常用的转染蛋白。染料标记的mRNA或疫苗载体已广泛用于转染研究。共聚焦显微镜和流式细胞术常用于评估细胞内微转染(如递送、摄取和翻译),共聚焦显微镜可更直观、准确地评估mRNA的翻译效率,交互式视频信息系统可用于评估全身或局部大转染(如分布、淋巴引流)。用于体内抗肿瘤活性评价的肿瘤模型主要包括治疗性或预防性皮下、原位和肺转移肿瘤模型,所建立的体内肿瘤模型应准确模拟人体病理。Bialkowski等人的研究表明TC-1肿瘤的TME可因肿瘤接种部位(即皮下、肺部和生殖道)的不同而有显著差异,这直接影响了E7-TriMix mRNA疫苗的抗肿瘤效果。在选择用于临床前评估的细胞或动物模型时,物种特异性是需要考虑的重要因素。为了获得更准确的评价信息,可以建立人源化动物模型。通过静脉注射人外周血单个核细胞(HLA-A2型)的CD34+造血干细胞建立的人源化小鼠,进入免疫缺陷NOD/ shi-scid IL-2Rγnull小鼠或C57BL/6小鼠,建立体内肿瘤模型(如结果表明,转染修饰的人CD133 mRNA的DCs处理人源化小鼠模型的中位生存期大于60天,转染修饰的小鼠CD133 mRNA的DCs处理同源小鼠肿瘤模型的中位生存期为38天。肿瘤疫苗理论上具有抗肿瘤复发和转移的作用,可以建立多种体内肿瘤模型进行评价,阐明这些疫苗在预防或治疗肿瘤转移或复发方面的优势。有效的抗肿瘤反应需要多个免疫细胞的协同作用,而不是单个细胞的作用。目前,大多数肿瘤疫苗的重点是诱导CD8+T细胞,但CD4+T细胞等免疫细胞在诱导和维持免疫记忆、增强CTLs的肿瘤杀伤作用中也发挥着重要作用。采用NKT配体(如α-GC)作为佐剂的mRNA Galsomes通过iNKT细胞诱导抗肿瘤作用。在含有CD4+和CD8+T细胞的培养系统中转染CD133 mRNA的DCs比仅含有CD4+或CD8+T细胞的培养系统中的DCs更有效地激活T细胞并杀死肿瘤靶细胞。也有人担心mRNA癌症疫苗的安全性和副作用。修饰后的mRNA可与血清蛋白结合形成血管闭塞,具有潜在的毒性。DOTAP/DP7-C mRNA具有良好的血清稳定性和较低的细胞毒性;然而,Lipo2000和PEI25K具有很强的细胞毒性。最后,我们期待mRNA癌症疫苗的成功临床转化。参考资料:He Q, Gao H, Tan D, Zhang H, Wang JZ. mRNA cancer vaccines: Advances, trends and challenges. Acta Pharm Sin B. 2022 Jul;12(7):2969-2989. doi: 10.1016/j.apsb.2022.03.011. Epub 2022 Mar 23. PMID: 35345451; PMCID: PMC8942458.
信使RNA疫苗上市批准紧急使用授权加速审批
100 项与 PRAME (Anocca) 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 肿瘤 | 临床前 | 瑞典 |  Anocca AB初创企业 Anocca AB初创企业 | 2024-07-11 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
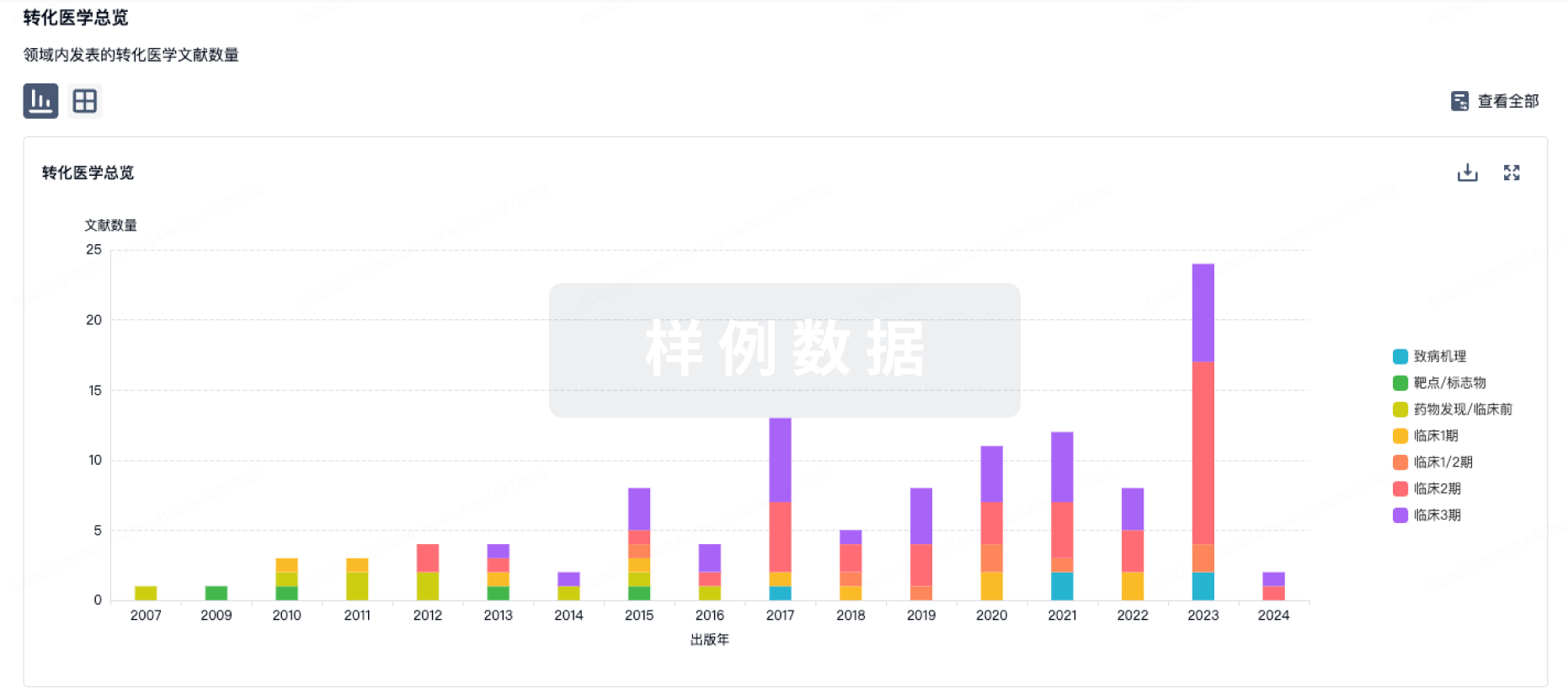
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
