更新于:2024-11-29
ASP-2016
更新于:2024-11-29
概要
基本信息
非在研机构- |
最高研发阶段临床1期 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评孤儿药 (美国) |
登录后查看时间轴
关联
1
项与 ASP-2016 相关的临床试验A Multicenter, Open-Label, Dose Escalation, Phase 1b Study to Evaluate the Safety, Tolerability and Preliminary Efficacy of ASP2016 for Friedreich Ataxia Cardiomyopathy
Friedreich Ataxia is a rare condition that causes damage to the nervous system and muscles. People with Friedreich Ataxia have difficulty walking, lose sensation in their arms and legs, and have slurred speech. It can also affect the heart and many people with Friedrich Ataxia develop serious heart problems. Friedreich Ataxia is a genetic condition which means a faulty gene is passed down through families. This type of gene therapy treats a genetic condition by providing a healthy copy of the gene.
At the time this study started, there was no approved treatment for heart problems in people with Friedreich Ataxia.
In this study, ASP2016 is being tested in humans for the first time. The people taking part are adults with Friedreich Ataxia who have heart problems.
The main aims of the study are to check the safety of ASP2016 and how people cope with (tolerate) ASP2016. ASP2016 is given as a slow injection into a vein. This is called an infusion. People will also take tablets of a medicine called prednisolone. This is taken to stop the immune system interfering with ASP2016.
Each person in the study will be given 1 single infusion of ASP2016. Different small groups will receive lower or higher doses of ASP2016. Each person will stay overnight in the clinic for at least 1 night after their infusion.
For the first few months, people will visit the clinic regularly. There may be the option of home visits by a study nurse at some visits. At the 6-month and 12-month visits extra tests, procedures, and scans will be done. One of these is an ECHO (echocardiogram) scan. This is like an ultrasound scan for the heart. Another is an endomyocardial biopsy. A tiny piece of their heart tissue is removed (biopsy). A flexible hollow tube (catheter) goes into the blood vessels up to the heart. Then, a small device on the end of the catheter takes a tiny piece of heart tissue (about the size of a pencil tip). Another is a cardiac MRI. This takes pictures of the inside of the heart using a powerful magnet. Another is a cardiopulmonary exercise test (CPET). This involves moving a specially designed set of bicycle pedals using hands and arms. This will check how the lungs, heart and muscles are affected during exercise.
After the 12-month visit, people will visit the clinic every few months for up to a few years.
At the time this study started, there was no approved treatment for heart problems in people with Friedreich Ataxia.
In this study, ASP2016 is being tested in humans for the first time. The people taking part are adults with Friedreich Ataxia who have heart problems.
The main aims of the study are to check the safety of ASP2016 and how people cope with (tolerate) ASP2016. ASP2016 is given as a slow injection into a vein. This is called an infusion. People will also take tablets of a medicine called prednisolone. This is taken to stop the immune system interfering with ASP2016.
Each person in the study will be given 1 single infusion of ASP2016. Different small groups will receive lower or higher doses of ASP2016. Each person will stay overnight in the clinic for at least 1 night after their infusion.
For the first few months, people will visit the clinic regularly. There may be the option of home visits by a study nurse at some visits. At the 6-month and 12-month visits extra tests, procedures, and scans will be done. One of these is an ECHO (echocardiogram) scan. This is like an ultrasound scan for the heart. Another is an endomyocardial biopsy. A tiny piece of their heart tissue is removed (biopsy). A flexible hollow tube (catheter) goes into the blood vessels up to the heart. Then, a small device on the end of the catheter takes a tiny piece of heart tissue (about the size of a pencil tip). Another is a cardiac MRI. This takes pictures of the inside of the heart using a powerful magnet. Another is a cardiopulmonary exercise test (CPET). This involves moving a specially designed set of bicycle pedals using hands and arms. This will check how the lungs, heart and muscles are affected during exercise.
After the 12-month visit, people will visit the clinic every few months for up to a few years.
开始日期2024-11-08 |
100 项与 ASP-2016 相关的临床结果
登录后查看更多信息
100 项与 ASP-2016 相关的转化医学
登录后查看更多信息
100 项与 ASP-2016 相关的专利(医药)
登录后查看更多信息
2
项与 ASP-2016 相关的新闻(医药)2024-10-08
As it works to rebuild from an earlier gene therapy setback, Astellas is doubling down on the one-and-done modality in a new deal with a London-based startup already in the clinic.
Astellas
said
Tuesday it will pay $30 million upfront and invest $20 million in AviadoBio in exchange for the chance to exclusively develop and commercialize the startup’s AAV-based gene therapy for people with frontotemporal dementia with progranulin mutations, or FTD-GRN. Investigators dosed the
first patient
with the experimental medicine, codenamed AVB-101, in April. The
Phase 1/2
also
opened in the US
in July.
Astellas could deliver up to $2.18 billion in license fees and milestone payments, the companies said. Astellas could develop AVB-101 for FTD-GRN and potentially other indications, the duo added. AviadoBio mentions ALS as another focus area for the startup, according to its website.
Patients with FTD, an early form of dementia, can die within three to 13 years after diagnosis, the companies said. Attention, memory, mobility, problem solving and other functions deteriorate in adults with the neurodegenerative disease.
AviadoBio’s gene therapy delivers a working copy of the GRN gene into the brain with the goal of halting the disease’s progress.
The Astellas investment provides a boost to AviadoBio, which does not appear to have raised another financing round since unveiling an
$80 million Series A
in December 2021.
Leading the upstart is former Novartis Gene Therapies chief business officer Lisa Deschamps.
“Together, we can further accelerate delivering this investigational medicine to families around the world who so desperately need treatment options for FTD-GRN and other neurological diseases,” Deschamps said in a press release, noting the company is completing dosing in the first cohort of the ASPIRE-FTD study.
The AviadoBio tie-up marks a further bet on gene therapy for Astellas, which ran into a major setback in 2020 and 2021, when
four boys died
after receiving the
company’s gene therapy
for a rare neuromuscular disease known as X-linked myotubular myopathy.
“People tend to look at the four boys’ deaths. And that’s a very unfortunate and tragic situation,” Astellas CEO Naoki Okamura
told Endpoints last month
. “On the other hand, there are healthier boys who received [the therapy] and survived for three to five years already. So we believe in the power of gene therapy to really make the difference.”
Astellas is
working with Kate Therapeutics
to improve the safety of the Audentes gene therapy, which is known as resamirigene bilparvovec, or AT132. The drugmaker also has Phase 1-stage gene therapies for Pompe disease (zocaglusagene nuzaparvovec, or AT845) and ASP2016 for cardiomyopathy associated with Friedreich’s ataxia. It also took a
write-down in April
on a preclinical Friedreich’s ataxia gene therapy known as AT808 from the Audentes buyout.
Meanwhile, multiple drugmakers are pulling back from gene therapy R&D, including Pfizer, while a
small number of startups
have been able to reel in large financing rounds to carry forward their work in the field.
The Japanese pharmaceutical company has a gene therapy R&D and manufacturing footprint in the Bay Area; Sanford, NC; and Tsukuba, Japan.

基因疗法并购临床1期
2024-09-04
近日,根据Biospace报道,Astellas(安斯泰来)公司旗下的Astellas Gene Therapies将关闭其位于旧金山的一家基因治疗工厂,并将基因治疗生产转移至北卡罗来州的另一家工厂。
受此影响,将裁员至少17人,影响数十人。根据安斯泰来发言人在一封邮件中所诉,该举动将会影响100名员工。
工厂关闭原因
Astellas Gene Therapies的发言人表示,该工厂正在关闭中,预计将于2025年3月完成。
在一份声明中写道,安斯泰来一直在优先考虑资源,并做出简化运营。在评估了其腺病毒的需求后,该公司决定关闭该设施,并将所有计划和项目转移至Astellas Gene Therapies位于北卡罗来州的另一家工厂。
尽管位于旧金山的工厂关闭、计划转移,但是Astellas Gene Therapies在加利福尼亚州的业务仍然存在。
今年5月,安斯泰来宣布在旧金山开设西海岸创新中心,占地约15万平方英尺,是安斯泰来基因治疗研究和开发业务的中心,包括Astellas Gene Therapies。
为何腺病毒需求下降?
在评估了其腺病毒的需求后,安斯泰来决定关闭一家大型生物制造工厂,说明安斯泰来对于腺病毒的需求下降。
在了解了安斯泰来在AAV基因治疗的失败史后,就明白安斯泰来确实是对腺病毒的需求不高了。
安斯泰来开始探足基因治疗可以追溯到2014年与哈佛大学的合作,到正式重点部署AAV基因治疗,是在2019年以30亿美元收购了Audentes公司。
然后,安斯泰来的噩梦就来了。
先是X-连锁肌小管性肌病(XLMTM)AAV基因疗法AT132,由于接连的临床死亡事件(在2020年5月、2020年6月、2020年8月、2021年9月累计报告了四例患者死亡严重不良事件),期间遭到FDA两度喊停。目前,小编还未查到AT132解除临床搁置的消息。
2022年4月,安斯泰来终止了来自Audentes的3条杜氏肌营养不良症(DMD)AAV临床前管线,AT702、AT751和AT753。
2022年6月,安斯泰来又一款AAV基因疗法AT845临床试验被FDA叫停,原因是一名接受AT845治疗的晚发型庞贝病患者报告了周围感觉神经病不良事件。该临床搁置于2023年1月解除。
延伸阅读:
30亿美元收购逐渐归零,安斯泰来又一款基因疗法被喊停
30亿美元收购败笔,安斯泰来终止多项基因治疗产品开发,累计5.6亿美元损失记账
AAV基因治疗的连续失败,以及多款管线的删减,安斯泰来对30亿美元收购Audentes已经进行了资产减值评估。
目前,在安斯泰来官网的管线图上,来自Audentes公司的AAV临床管线仅有3条,分别是妾身未明的AT132(临床2期)、身具前科的AT845(临床1/2期)、以及一款新提的弗里德赖希共济失调相关心肌疾病项目ASP2016(临床1期)。其它基因治疗项目还未推进临床。
安斯泰来的基因治疗项目(图片来源:参考资料2)
如果除去AT132,也就仅有两款AAV产品需要生产临床制剂,因为都处于早期临床,产量要求也不高,因此,对于腺病毒载体的需求就下降了。
小结
除了AAV基因治疗,细胞治疗也是安斯泰来看好的方向。为了加强自身在细胞治疗领域的地位,安斯泰来于2018年收购了Universal Cells,但是在2023年终止与Adaptimmune公司的合作中,似乎暴露出Universal Cells的干细胞技术平台可能存在问题。
感觉安斯泰来在细胞治疗上,也岌岌可危......
延伸阅读:终止TCR-T合作,安斯泰来艰难的CGT之旅
(本文仅代表作者观点,不代表平台立场)
参考资料:
1.https://www.biospace.com/job-trends/astellas-gene-therapies-to-close-biomanufacturing-facility-affecting-about-100-employees
2.https://www.astellas.com/en/innovation/pipeline
本周好文推荐
如需转载请联系佰傲谷并在醒目位置注明出处
·
·
·
·
·
·
·
·
基因疗法并购
100 项与 ASP-2016 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 心肌疾病 | 临床1期 | 美国 | 2024-11-08 | |
| Friedreich共济失调 | 临床1期 | 美国 | 2024-11-08 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
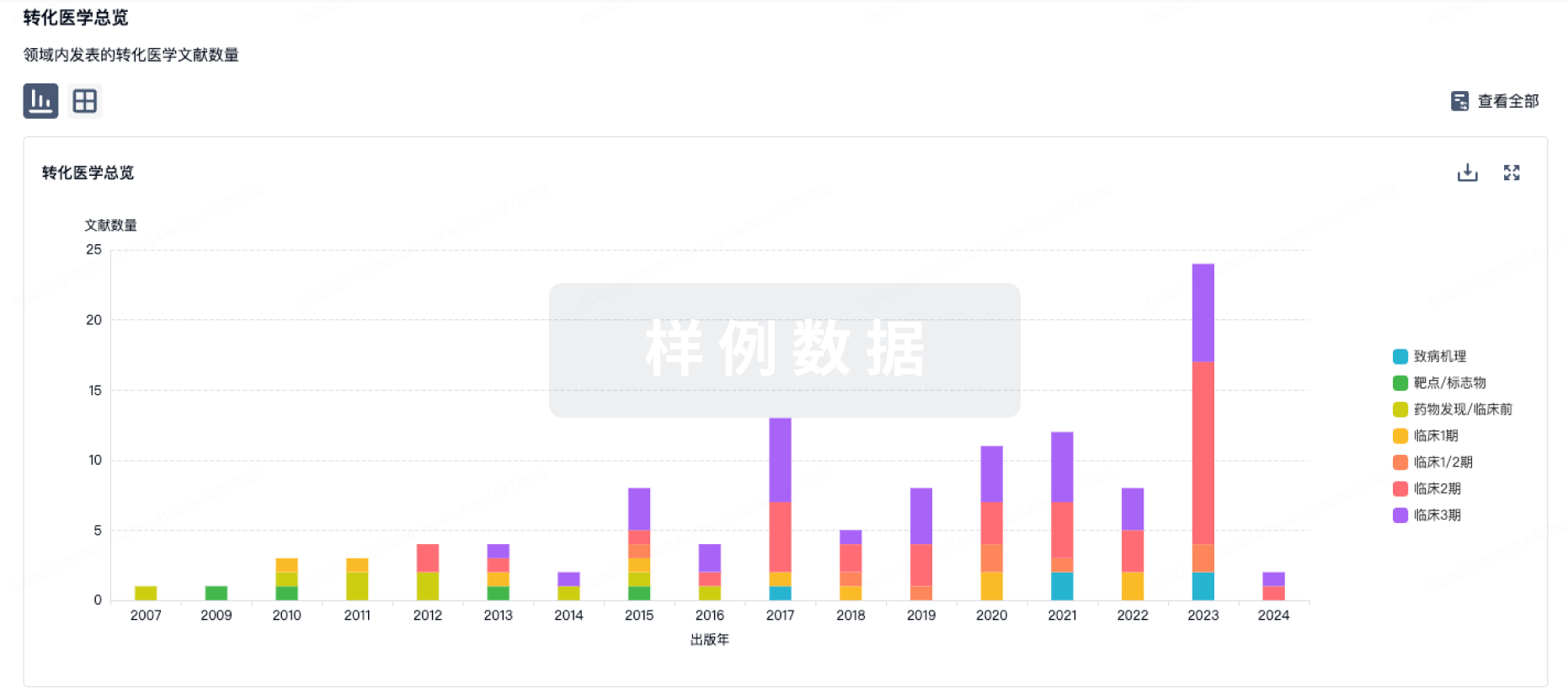
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
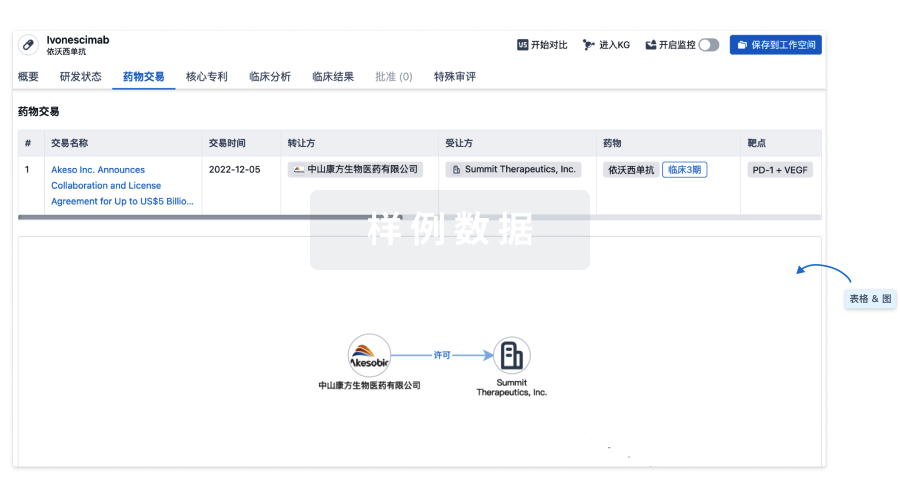
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
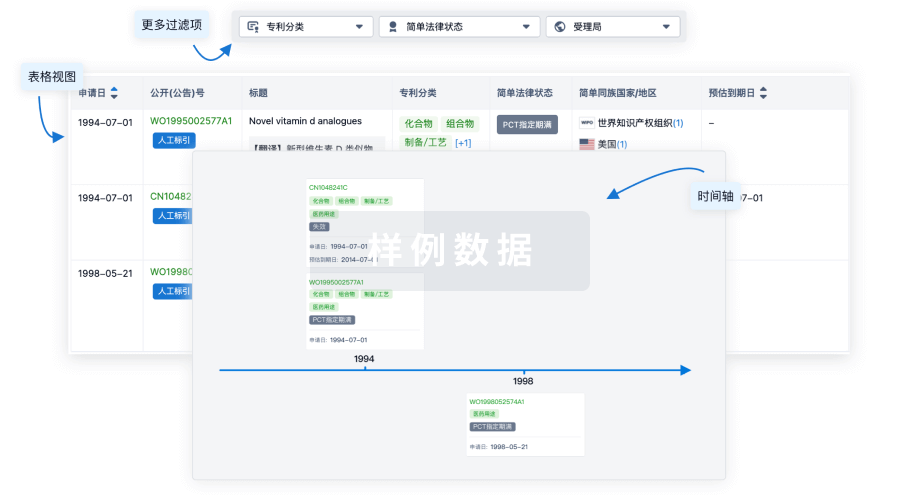
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
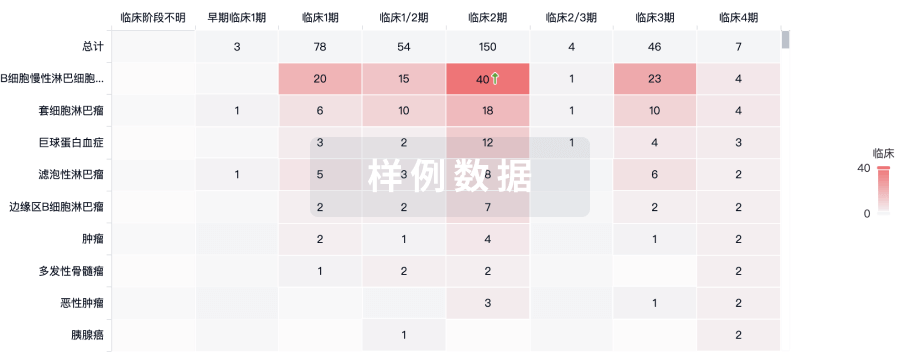
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

