预约演示
更新于:2025-01-23
L-14
更新于:2025-01-23
概要
基本信息
结构/序列
分子式C24H29N3O2 |
InChIKeyQMGIYQLIQGUDJE-UHFFFAOYSA-N |
CAS号332939-38-5 |
关联
1
项与 L-14 相关的临床试验KCT0009813
A 12-week, Randomized, Double-blind, Placebo-Controlled Clinical Trial for the Evaluation of the Efficacy and Safety of L14 on Reducing Body Fat
开始日期2024-08-21 |
100 项与 L-14 相关的临床结果
登录后查看更多信息
100 项与 L-14 相关的转化医学
登录后查看更多信息
100 项与 L-14 相关的专利(医药)
登录后查看更多信息
18
项与 L-14 相关的文献(医药)2023-12-14·Journal of Medicinal Chemistry1区 · 医学
Discovery of Novel 8-Hydroxyquinoline Derivatives with Potent In Vitro and In Vivo Antifungal Activity
1区 · 医学
Article
作者: Wu, Hao ; Shen, Fuming ; Li, Liping ; Ni, Tingjunhong ; Jiang, Yuanying ; Yan, Lan ; Wang, Jiayin ; Fang, Ting ; Zhang, Dazhi ; Lu, Hui ; Ji, Zhe
2023-07-28·Antioxidants (Basel, Switzerland)
Ameliorative Effects of Lactobacillus paracasei L14 on Oxidative Stress and Gut Microbiota in Type 2 Diabetes Mellitus Rats.
Article
作者: Chen, Shangwu ; Yang, Yi ; Zhong, Xinxin ; Tong, Xiaoling ; Dai, Fangyin ; Zeng, Zhu
2013-05-20·Inorganic Chemistry2区 · 化学
Integrated and Passive 1,2,3-Triazolyl Groups in Fluorescent Indicators for Zinc(II) Ions: Thermodynamic and Kinetic Evaluations
2区 · 化学
Article
作者: Morris, Deborah R. ; Zhu, Lei ; Davidson, Michael W. ; Levenson, Cathy W. ; Simmons, J. Tyler ; Allen, John R. ; Clark, Ronald J.
100 项与 L-14 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 念珠菌病 | 临床前 | 中国 | 2023-11-17 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
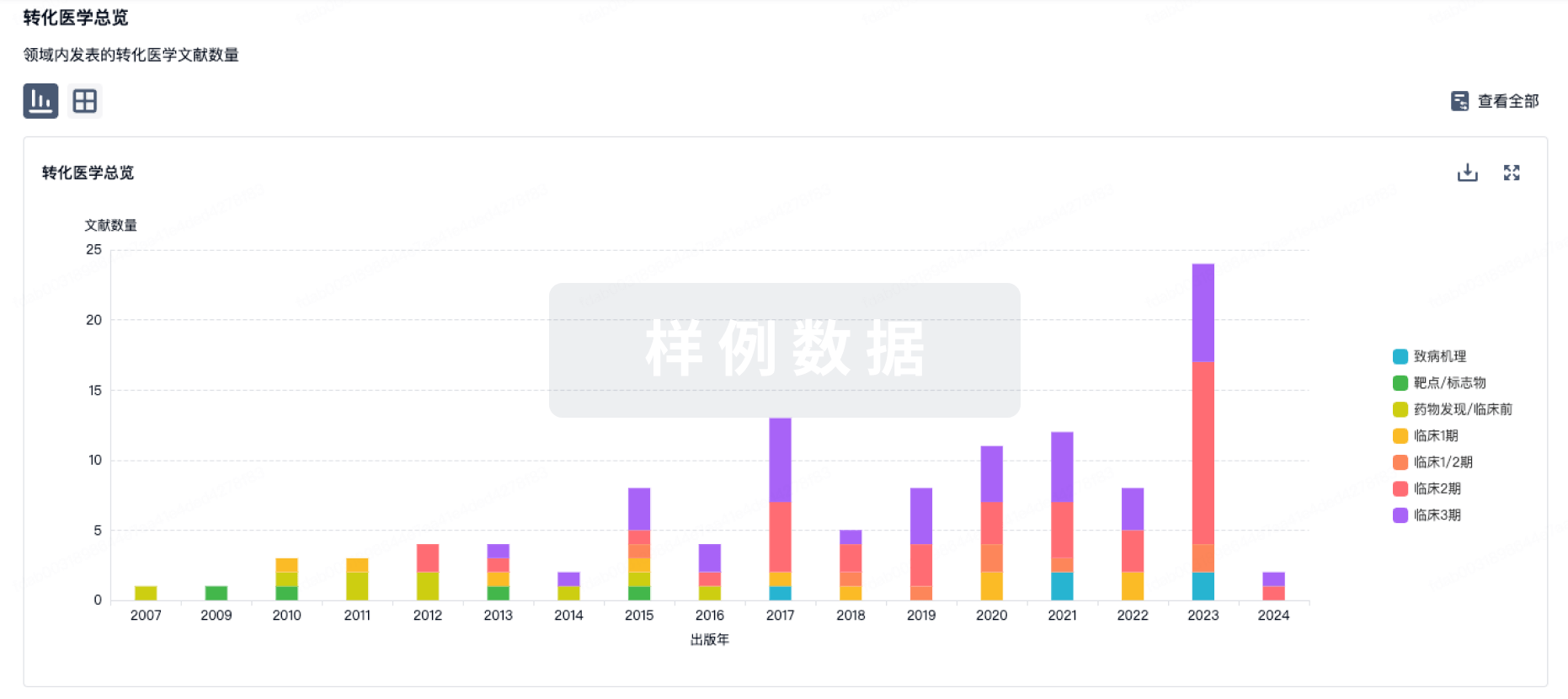
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
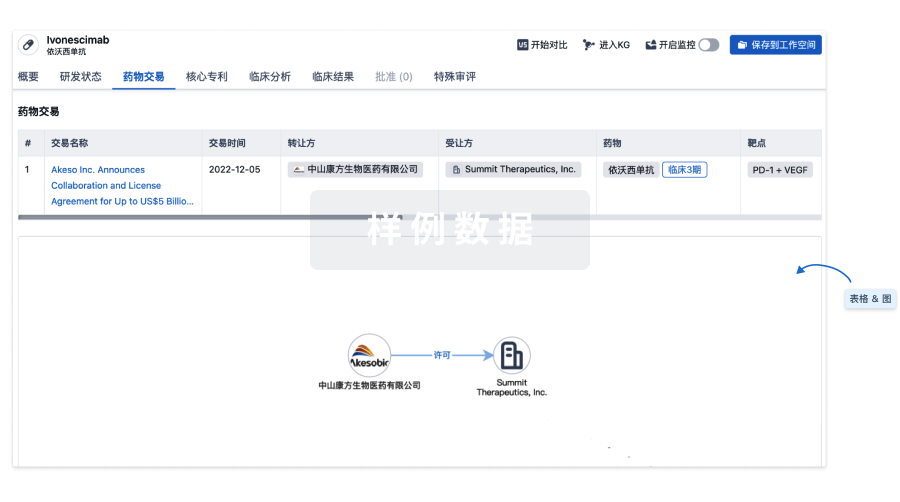
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
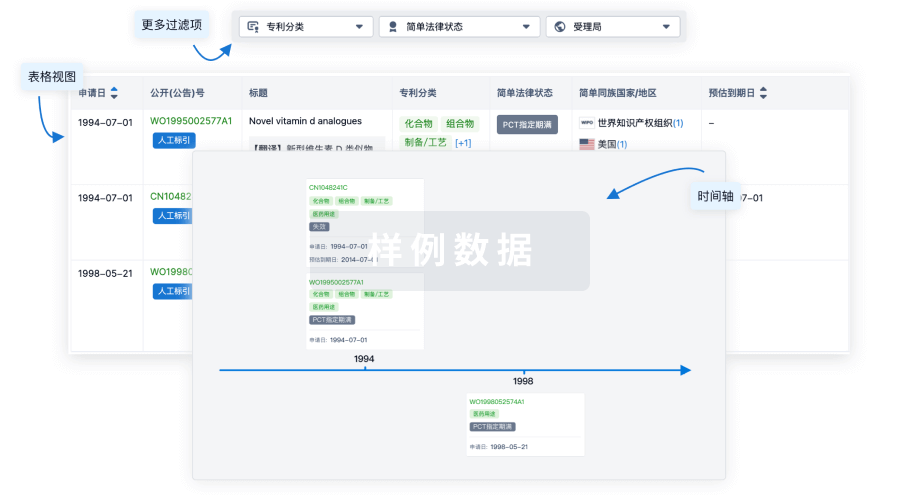
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
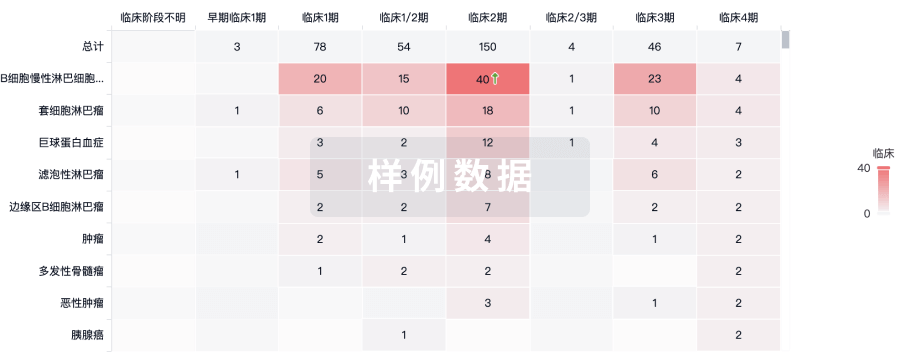
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

