更新于:2024-12-14
Aldesleukin biosimilar(AMEGA Biotech)
阿地白介素生物类似药(AMEGA Biotech)
更新于:2024-12-14
概要
基本信息
原研机构 |
在研机构 |
非在研机构- |
最高研发阶段临床阶段不明 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
序列信息
Sequence Code 720

当前序列信息引自: *****
关联
6
项与 阿地白介素生物类似药(AMEGA Biotech) 相关的临床试验Prophylaxis Roles of Low Dose of IL-2 on Chronic Graft-versus-host Disease After Haploidentical Transplantation
开始日期2020-06-20 |
申办/合作机构 |
A perspective, multicenter, open-label, randomized, controlled trial for DCAG combined with unrelated umbilical cord blood stem cell microtransplantation and IL-2 treatment in elderly patients with newly diagnosed acute myeloid leukemia
开始日期2019-07-01 |
申办/合作机构- |
Low-dose IL-2 to expand endogenous regulatory T cells and achieve tolerance in liver transplantation - LITE
开始日期2017-09-21 |
申办/合作机构 |
100 项与 阿地白介素生物类似药(AMEGA Biotech) 相关的临床结果
登录后查看更多信息
100 项与 阿地白介素生物类似药(AMEGA Biotech) 相关的转化医学
登录后查看更多信息
100 项与 阿地白介素生物类似药(AMEGA Biotech) 相关的专利(医药)
登录后查看更多信息
13,299
项与 阿地白介素生物类似药(AMEGA Biotech) 相关的文献(医药)2025-02-01·IMMUNOLOGY LETTERS
Rescuing pathogen-specific memory B-cell from PBMC of prior Zika virus-infected individuals
Article
作者: Gonçalves Fonseca, Simone ; Fontinele, Hitallo Guilherme Costa ; Silva, Jacyelle Medeiros ; França, Renato Kaylan Alves de Oliveira ; Fonseca, Simone Gonçalves ; Barros, Pedro Henrique ; Brigido, Marcelo Macedo ; Maranhão, Andrea Queiroz
Immunological memory, a fundamental immune system mechanism, is instrumental in long-term protection. Successful vaccines can elicit and sustain immunological memory against pathogens for the long term. Memory B cells (MBC) are key players in secondary responses due to their longevity and rapid differentiation into high-affinity antibody-secreting cells upon second antigen exposure. However, the availability of circulating MBCs is limited. Here we describe a protocol, which presents a straightforward and practical method for activating and expanding Zika virus (ZIKV) specific MBC. PBMCs collected from individuals who had been infected with ZIKV two years prior were cultured by supplementing with IL-2 and R848, a TLR-7/8 agonist, and then pulsed with inactivated virus. After seven days, this stimulation led to a notable rise in virus-specific functional MBC, as evidenced by a significant increase in the production of anti-ZIKV IgG. Importantly, the ZIKV pulse did not induce changes in the PBMC culture of individuals without a history of ZIKV infection. These findings demonstrate that virus-specific MBC can be expanded in vitro, even using PBMC cultures from individuals infected years before. Therefore, our protocol is a practical and effective tool for studies that require a larger number of human MBCs from previously infected individuals that are functional and specific to the pathogen under investigation.
2025-01-01·EUROPEAN JOURNAL OF PHARMACOLOGY
An antibody against human interleukin-2 showing minimal binding to neonatal Fc receptor: Potent anti-tumor activity and reduced systemic toxicity in mice
Article
作者: Lv, Yunying ; Wang, Chao ; Lou, Jing ; Chen, Jianhe ; Jin, Zheng
The anti-tumor efficacy of immune checkpoint inhibitors can be enhanced by combining them with interleukin-2 (IL-2), which promotes the clonal expansion of antigen-activated CD8+ T cells and natural killer cells. However, IL-2 can simultaneously bind to endothelial cells and regulatory T cells to induce adverse and immunosuppressive effects. Such off-target binding can be inhibited by co-administering IL-2 with anti-IL-2 antibodies, but these antibodies can interact with neonatal Fc receptor to protect the IL-2 from lysosomal degradation, leading to substantial toxicity. Here we developed and humanized a mouse monoclonal antibody against human IL-2 and introduced two mutations (H310A and H435Q) in the Fc segment in order to weaken binding to the neonatal Fc receptor. The humanized antibody bound tightly to IL-2 but minimally to neonatal Fc receptor. Hydrogen deuterium exchange mass spectrometry indicated that the antibody binds to IL-2 at the site where the cytokine binds to subunit α (CD25) of the trimeric IL-2 receptor. Co-administering the antibody with human IL-2 improved the cytokine's ability to slow growth of two types of colorectal tumor xenografts (CT26, MC38) in immunocompetent mice. Co-administration to mice with CT26 xenografts strongly expanded CD8+ T cells, without expanding regulatory T cells in spleen or blood. Co-administration to mice with MC38 xenografts downregulated PD-1 and CD44, while upregulating CD62L and CD69, on tumor-infiltrating CD8+ T cells. Notably, co-administration synergized with anti-PD-1 antibody to slow growth of MC38 xenografts. Our results suggest that the combination of human IL-2 and our novel antibody shows promise for providing more effective, less toxic cancer immunotherapy.
2025-01-01·INTERNATIONAL JOURNAL OF BIOLOGICAL MACROMOLECULES
Novel design of potent anti-tumour activity of IL-2 prodrug by FAPα-mediated activation
Article
作者: Wu, Hang ; Zhou, Xi ; Zhang, Yunxiao ; Wang, Zhaofeng ; Zhao, Wenjie ; Peng, Dezheng ; Zhu, Bo ; Si, Linlin ; Yao, Songjin ; Yu, Yunzhi ; Liu, Yu ; Liu, Qiuyue
Interleukin-2 (IL-2) is a T cell growth factor that is essential for the proliferation of T cells and the generation of effector and memory cells. The antitumor activity of high-dose IL-2 therapy requires maintaining the affinity between IL-2 and IL2-Rα, which can also bring serious toxic side effects. To address this issue, we designed ZGP-Cysteamine-IL-2-K64C and (ZGP-Cysteamine)2-IL-2-(K43C, K64C) based on the strategy of FAPα enzyme-activated prodrugs, and investigated their anti-tumour activity and side effects. In vitro FAPα enzyme cleavage results indicated that the side-chain modified ZGP-Cysteamine moiety could be precisely recognized and cleaved by FAPα, thereby restoring the activity of native IL-2 capable of binding to IL-2Rα in the tumour microenvironment, where it promotes the expansion of CD8+ T cells. Meanwhile, surface plasmon resonance analysis revealed that, compared to wt-IL-2, both ZGP-Cysteamine-IL-2-K64C and (ZGP-Cysteamine)2-IL-2-(K43C, K64C) exhibited significantly reduced affinity for IL-2Rα, while their affinity for IL-2Rβγ remained unchanged. Remarkably, ZGP-Cysteamine-IL-2-K64C and (ZGP-Cysteamine)2-IL-2-(K43C, K64C) almost completely eliminated the pulmonary edema and vascular permeability. Furthermore, the combination of ZGP-Cysteamine-IL-2-K64C and PD-1 blockade showed robust anti-tumour activity in mice tumour models. Our study provides new insights into the structural design of IL-2 prodrug with low side effect and robust anti-tumour efficacy.
132
项与 阿地白介素生物类似药(AMEGA Biotech) 相关的新闻(医药)2024-12-13
Single-agent MDNA11 was more effective than a combination of immune checkpoint inhibitors (anti-mPD1 and anti-mCTLA4) in preventing metastasis and achieving long-term survival in an aggressive mouse model of triple negative breast cancer (TNBC) Mice treated with MDNA11 prior to surgery were able to mount powerful immune and memory response to subsequent tumor rechallenges, demonstrating potential to prevent new tumor growth TORONTO and HOUSTON, Dec. 13, 2024 (GLOBE NEWSWIRE) -- Medicenna Therapeutics Corp. (“Medicenna” or the “Company”) (TSX: MDNA, OTCQX: MDNAF), a clinical-stage immunotherapy company focused on the development of Superkines, announced that new preclinical data on MDNA11 was presented at the 2024 San Antonio Breast Cancer Symposium (SABCS), the world’s largest breast cancer conference, taking place from December 10-13, 2024, in San Antonio, Texas. Metastasis is the leading cause of cancer-related deaths worldwide. 1 in 8 women will be diagnosed with breast cancer in the US and 1 in 3 of those will become metastatic resulting in approximately 42,000 breast cancer deaths every year. Of those deaths, it is estimated that 97-99% of those will be from metastatic breast cancer.1 Among breast cancer subtypes, triple-negative breast cancer (TNBC) is particularly aggressive, spreading more rapidly and representing a significant unmet medical need. Neoadjuvant immunotherapy offers a promising treatment strategy in which patients with advanced but resectable cancer receive immunotherapy before surgery. This approach aims to shrink tumors, making them easier to remove, delay or prevent metastasis, while also targeting cancerous tissue that may not yet be detectable. Additionally, immunotherapy can prime the immune system against tumor antigens, promoting the development of long-lasting anti-cancer T cells. These T cells may help protect against residual tumor cells that could re-emerge and drive metastatic disease. The presentation at 2024 SABCS highlighted the potential of MDNA11, a long-acting ‘β-enhanced not-α’ IL-2 Superkine, to improve treatment outcomes when administered prior to surgery. New findings demonstrated that a single low dose of MDNA11 as a neoadjuvant therapy significantly prevented metastasis, extended survival, and enabled a memory immune response that protects against tumor rechallenges in a TNBC model. “We are impressed by MDNA11’s remarkable capability, in the on-going ABILITY-1 clinical trial, to durably control cancer in patients with advanced late-stage metastatic disease in immunotherapy resistant tumors. This naturally encourages us to evaluate the potential of MDNA11 to tackle cancer head-on at the earlier stages of the disease and reverse cancer from a death sentence to a chronic manageable condition,” said Fahar Merchant, PhD, President and CEO of Medicenna. “Our findings show that MDNA11 alone not only prevents metastasis in an aggressive preclinical breast cancer model when administered as a single pre-treatment prior to surgery, but also delivers superior long-term survival by preventing metastasis when compared to combinations of the leading checkpoint inhibitors, such as anti-CTLA4 and anti-PD-1. These data illustrate MDNA11’s potential to redefine traditional immunotherapy by attacking cancer at its earliest stages and efficiently leverage the patient’s healthier immune status to dramatically improve patient outcomes.” Key findings from presentation of MDNA11 pre-treatment prior to surgery in the aggressive TNBC model included: Metastasis Prevention: A single dose of neoadjuvant MDNA11 prevented the spread of tumors, with vast majority of treated mice (7/8 at high-dose of 5 mg MDNA11/kg and 6/7 at low-dose of 2 mg MDNA11/kg) surviving to study end (>4 months) without signs of metastasis despite tumor rechallenges. By contrast, all mice in the control group developed multiple metastasis and died within ~2 months even after tumor resection surgery.Superior to Checkpoint Inhibitors: MDNA11 alone, even at the lower dose, was more effective than a combination of immune checkpoint inhibitors (anti-mPD1 and anti-mCTLA4) in preventing metastasis and achieving long-term survival.Memory Response Against Tumor Rechallenge: Mice treated with MDNA11 were able to mount strong immune response to subsequent tumor rechallenges by engaging tumor antigen-specific T cells, demonstrating protection against new tumor growth.Immune Cell Activation: MDNA11 promoted the infiltration of CD8+ T cells with potent tumor-killing capacity (GrzB+) into the tumor microenvironment (TME) with no impact on immune-suppressive Treg cells, driving a potent and lasting immune response. A copy of the presentation has been posted on the “Scientific Presentations” page of Medicenna’s website. About MDNA11 MDNA11 is an intravenously administered, long-acting, ‘beta-enhanced not-alpha’ IL-2 Superkine specifically engineered to overcome the shortcomings of aldesleukin and other next generation IL-2 variants by preferentially activating immune effector cells (CD8+ T and NK cells) responsible for killing cancer cells, with minimal or no stimulation of immunosuppressive Tregs. These unique proprietary features of the IL-2 Superkine have been achieved by incorporating seven specific mutations and genetically fusing it to a recombinant human albumin scaffold to improve the pharmacokinetic (PK) profile and pharmacological activity of MDNA11 due to albumin’s natural propensity to accumulate in highly vascularized sites, in particular tumor and tumor draining lymph nodes. MDNA11 is currently being evaluated in the Phase 1/2 ABILITY-1 study as both monotherapy and in combination with pembrolizumab. About Medicenna Therapeutics Medicenna is a clinical-stage immunotherapy company focused on developing novel, highly selective versions of IL-2, IL-4 and IL-13 Superkines and first-in-class Empowered Superkines. Medicenna’s long-acting IL-2 Superkine, MDNA11, is a next-generation IL-2 with superior affinity toward CD122 (IL-2 receptor beta) and no CD25 (IL-2 receptor alpha) binding, thereby preferentially stimulating cancer-killing effector T cells and NK cells. MDNA11 is being evaluated in the Phase 1/2 ABILITY-1 Study (NCT05086692) as a monotherapy and in combination with pembrolizumab. Medicenna’s IL-4 Empowered Superkine, bizaxofusp (formerly MDNA55), has been studied in 5 clinical trials enrolling over 130 patients, including a Phase 2b trial for recurrent GBM, the most common and uniformly fatal form of brain cancer. Bizaxofusp has obtained FastTrack and Orphan Drug status from the FDA and FDA/EMA, respectively. Medicenna’s early-stage high-affinity IL-2β biased IL-2/IL-15 Super-antagonists, from its MDNA209 platform, are being evaluated as potential therapies for autoimmune and graft-versus host diseases. Medicenna’s early-stage BiSKITs™ (Bifunctional SuperKine ImmunoTherapies) and the T-MASK™ (Targeted Metalloprotease Activated SuperKine) programs are designed to enhance the ability of Superkines to treat immunologically “cold” tumors. For more information, please visit www.medicenna.com, and follow us on Twitter and LinkedIn. Forward-Looking Statements This news release may contain forward-looking statements within the meaning of applicable securities laws. Forward-looking statements include, but are not limited to, express or implied statements regarding the future operations of the Company, estimates, plans, strategic ambitions, partnership activities and opportunities, objectives, expectations, opinions, forecasts, projections, guidance, outlook or other statements that are not historical facts, such as statements on the therapeutic treatment potential and safety profile of MDNA11 (both as monotherapy and in combination with pembrolizumab) and the timing and/or release of any additional clinical updates. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early-stage pre-clinical or clinical studies may not be indicative of full results or results from later stage or larger scale clinical studies and do not ensure regulatory approval. You should not place undue reliance on these statements, or the scientific data presented. Forward-looking statements are often identified by terms such as “will”, “may”, “should”, “anticipate”, “expect”, “believe”, “seek”, “potentially” and similar expressions. and are subject to risks and uncertainties. Forward-looking statements are based on a number of assumptions believed by the Company to be reasonable at the date of this news release. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, there can be no assurance that such statements will prove to be accurate. These statements are subject to certain risks and uncertainties and may be based on assumptions that could cause actual results and future events to differ materially from those anticipated or implied in such statements. Important factors that could cause actual results to differ materially from the Company’s expectations include the risks detailed in the latest annual information form of the Company and in other filings made by the Company with the applicable securities regulators from time to time in Canada. The reader is cautioned that assumptions used in the preparation of any forward-looking information may prove to be incorrect. Events or circumstances may cause actual results to differ materially from those predicted, as a result of numerous known and unknown risks, uncertainties, and other factors, many of which are beyond the control of the Company. The reader is cautioned not to place undue reliance on any forward-looking information. Such information, although considered reasonable by management, may prove to be incorrect and actual results may differ materially from those anticipated or implied in forward-looking statements. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The forward-looking statements contained in this news release are made as of the date hereof and except as required by law, we do not intend and do not assume any obligation to update or revise publicly any of the included forward-looking statements. This news release contains hyperlinks to information that is not deemed to be incorporated by reference in this new release. Investor and Company Contact: Christina CameronInvestor Relationsir@medicenna.com(647) 953-0673 Daniel ScarrInvestor Relations & Corporate Developmentdscarr@medicenna.com(647) 220-4509 1 Metastatic breast cancer statistics, METAvivor. https://www.metavivor.org/mbc-prep/metastatic-breast-cancer-statistics
免疫疗法孤儿药临床2期临床结果
2024-12-05
70-year-old patient with advanced chemo-refractory anal cancer achieves complete response (CR) in 8 weeks when treated with MDNA11 in combination with Merck’s (known as MSD outside of the US and Canada) anti-PD-1 therapy, KEYTRUDA® (pembrolizumab) Complete regression of all tumors in two CPI-resistant patients in monotherapy arms continue to show durability with a patient with melanoma remaining tumor free at week 63 while a patient with pancreatic cancer remains off all anti-cancer therapy for 11 months after completing the study A patient with advanced MSS colorectal cancer with a previously announced partial response (PR) continues treatment in the combination escalation arm as of week 32 with a confirmed PR Both objective responses (1 CR and 1PR) among evaluable patients in the combination dose escalation arm (N=9) are in tumor types with high unmet need and where checkpoint inhibitors (CPI) have not been approved The ABILITY-1 study is showing promising disease control rates (DCR = CR+PR+SD) of 55% (1 CR, 4 PRs, and 6 SDs) and 78% (1 CR, 1 PR and 5 SDs) in the monotherapy and combination arms, respectfully
Additional monotherapy and combination clinical data from the ABILITY-1 study will be presented at medical conferences in Q1 and Q2 of 2025 TORONTO and HOUSTON, Dec. 05, 2024 (GLOBE NEWSWIRE) -- Medicenna Therapeutics Corp. (“Medicenna” or the “Company”) (TSX: MDNA, OTCQX: MDNAF), a clinical-stage immunotherapy company focused on the development of Superkines, announced that updated clinical data from the ongoing Phase 1/2 ABILITY-1 study were presented today at the 2024 Immunotherapy Bridge held in Naples, Italy. The oral presentation by Dr Arash Yavari, MB BS, DPhil, included new data highlighting the first complete response (CR) in the combination dose escalation phase of the study in a 70-year-old patient with advanced anal squamous cell carcinoma (anal SCC), in addition to follow up safety and efficacy results from the monotherapy and combination arms of the ABILITY-1 study. The anal SCC patient previously progressed on two prior lines of treatment comprising of chemotherapy combination and chemo-radiation treatments. The patient achieved a 100% reduction of all measurable target and non-target lesions by Week 8, highlighting MDNA11’s potential to enhance checkpoint inhibitor efficacy in advanced solid tumor types traditionally considered less sensitive to immunotherapy. “These remarkable results from our monotherapy and early data from the combination dose escalation highlights the transformative potential of MDNA11 as a combination therapy with checkpoint inhibitors like KEYTRUDA®, while demonstrating an acceptable safety profile as dose escalation continues,” said Fahar Merchant, PhD, President and CEO of Medicenna. “Achieving a complete response in a patient with anal squamous cell carcinoma, a cancer with historically low immunotherapy response rates, demonstrates MDNA11’s ability to reinvigorate the immune system and tackle difficult-to-treat tumors. We look forward to sharing additional clinical data at medical conferences in Q1 and Q2 of 2025 as we advance MDNA11 as a potent and safe immunotherapy to dramatically improve current immunotherapies and deliver life-changing outcomes for patients.” Key findings from the on-going ABILITY-1 study (data cut-off as of November 18th, 2024) include: Safety Profile MDNA11 has a favorable safety profile both as a monotherapy and in combination with KEYTRUDA®. Over 90% of treatment-related adverse events (TRAEs) were Grade 1-2 and transient, with no DLTs or new safety signals observed in combination dose escalation cohorts. MDNA11 at doses of 120 µg /kg (Q3W monotherapy; Q2W and Q3W in combination with Keytruda (400 mg Q6W) are currently being evaluated. Immunodynamics MDNA11 preferentially expands immune effector cells, including activated (CD25+) and “stemness-like” (TCF-1+) CD8+ T cells, which are critical for sustained anti-tumor responses.Robust, dose-dependent lymphocyte expansion in combination with KEYTRUDA®, sustained with repeat MDNA11 dosing. Monotherapy Tumor Response in Immune Checkpoint Inhibitor-Resistant Patients MDNA11 continues to demonstrate encouraging deep and durable single-agent anti-tumor activity among patients who progressed on prior ICI therapy: An objective response rate (ORR) of 30% in the monotherapy dose expansion arm with 3 PRs among 10 patients who had all previously failed ICI therapy and had advanced and/or metastatic melanoma, non-melanoma skin cancer or MSI-H/dMMR tumors. The ORR is 25% from a total of 20 patients when including 10 phase 2 eligible patients from the MDNA11 monotherapy dose escalation arm who received at least 60 µg/kg MDNA11 and were ICI-resistant.Overall, objective responses in ICI-resistant patients include 1 CR and 4 PRs 2 PRs among 3 MSI-H patients (ORR of 66.7%) with both responders having metastatic pancreatic ductal adenocarcinoma (PDAC).1 CR and 2 PRs among 11 patients with cutaneous melanoma (ORR of 27.3%). Complete resolution of all target and non-target lesions in two patients: 1 confirmed CR in a melanoma patient continues on treatment as of week 63.Pancreatic cancer patient (MSI-H) achieved complete resolution of target and non-target metastatic lesions in the liver including a new lesion treated with MDNA11 following a single cycle of radiation, achieved durable response for 20 months during the study and continues to remain off anti-cancer therapy for 11 months. SD in 6 patients for a disease control rate (DCR = CR+PR+SD) of 55% including 3 with duration > 6 months, yielding a clinical benefit rate of 40% (8/20). MDNA11 Combination with KEYTRUDA® Tumor Response The objective of the combination dose escalation/evaluation portion of the ABILITY-1 study is to assess the safety, pharmacokinetics and immunodynamics of MDNA11 at various doses and dosing regimens when combined with KEYTRUDA® (400mg, Q6W), and to determine the recommended dose and schedule for the combination dose expansion portion of the study. Encouraging preliminary signs of anti-tumor activity have been observed with MDNA11 in combination with KEYTRUDA® to date in dose escalation cohorts 1 (60 µg/kg Q2W MDNA11) and 2 (90 µg/kg Q2W MDNA11). Among 9 heavily pre-treated, efficacy-evaluable patients, tumor control was observed in 7 patients for a DCR of 78% including a CR in an anal squamous cell carcinoma patient and PR in a microsatellite-stable (MSS) colorectal cancer patient. CR in a 70-year-old male with anal squamous cell carcinoma Patient progressed on 2 prior lines of chemotherapy (1L capecitabine/mitomycin plus radiation therapy; 2L carboplatin/paclitaxel)CR achieved on first study evaluable imaging scan at Week 8; patient continues on combination treatment Confirmed PR in 52-year-old female with MSS colorectal cancer Patient progressed on 2 prior lines of chemotherapy (1L folinate/fluorouracil/oxaliplatin; 2L capecitabine)Continues on combination treatment as of week 32
A copy of the presentation has been posted on the “Scientific Presentations” page of Medicenna’s website. About MDNA11 MDNA11 is an intravenously administered, long-acting, ‘beta-enhanced not-alpha’ IL-2 Superkine specifically engineered to overcome the shortcomings of aldesleukin and other next generation IL-2 variants by preferentially activating immune effector cells (CD8+ T and NK cells) responsible for killing cancer cells, with minimal or no stimulation of immunosuppressive Tregs. These unique proprietary features of the IL-2 Superkine have been achieved by incorporating seven specific mutations and genetically fusing it to a recombinant human albumin scaffold to improve the pharmacokinetic (PK) profile and pharmacological activity of MDNA11 due to albumin’s natural propensity to accumulate in highly vascularized sites, in particular tumor and tumor draining lymph nodes. MDNA11 is currently being evaluated in the Phase 1/2 ABILITY-1 study as both monotherapy and in combination with KEYTRUDA®. About the ABILITY-1 Study The ABILITY-1 study (NCT05086692) is a global, multi-center, open-label study that assesses the safety, tolerability, pharmacokinetics, pharmacodynamics and anti-tumor activity of MDNA11 as monotherapy or in combination with KEYTRUDA®. In the combination dose escalation portion of the Phase 2 study, approximately 20 patients are expected to be enrolled and administered ascending doses of MDNA11 intravenously in combination with KEYTRUDA®. This portion of the study includes patients with a wide range of solid tumors with the potential for susceptibility to immune modulating therapeutics. Upon identification of an appropriate dose regimen for combination, the study will proceed to combination dose expansion. About Medicenna Therapeutics Medicenna is a clinical-stage immunotherapy company focused on developing novel, highly selective versions of IL-2, IL-4 and IL-13 Superkines and first-in-class Empowered Superkines. Medicenna’s long-acting IL-2 Superkine, MDNA11, is a next-generation IL-2 with superior affinity toward CD122 (IL-2 receptor beta) and no CD25 (IL-2 receptor alpha) binding, thereby preferentially stimulating cancer-killing effector T cells and NK cells. MDNA11 is being evaluated in the Phase 1/2 ABILITY-1 Study (NCT05086692) as a monotherapy and in combination with KEYTRUDA®. Medicenna’s IL-4 Empowered Superkine, bizaxofusp (formerly MDNA55), has been studied in 5 clinical trials enrolling over 130 patients, including a Phase 2b trial for recurrent GBM, the most common and uniformly fatal form of brain cancer. Bizaxofusp has obtained FastTrack and Orphan Drug status from the FDA and FDA/EMA, respectively. Medicenna’s early-stage high-affinity IL-2β biased IL-2/IL-15 Super-antagonists, from its MDNA209 platform, are being evaluated as potential therapies for autoimmune and graft-versus host diseases. Medicenna’s early-stage BiSKITs™ (Bifunctional SuperKine ImmunoTherapies) and the T-MASK™ (Targeted Metalloprotease Activated SuperKine) programs are designed to enhance the ability of Superkines to treat immunologically “cold” tumors. For more information, please visit www.medicenna.com, and follow us on Twitter and LinkedIn. Forward-Looking Statements This news release may contain forward-looking statements within the meaning of applicable securities laws. Forward-looking statements include, but are not limited to, express or implied statements regarding the future operations of the Company, estimates, plans, strategic ambitions, partnership activities and opportunities, objectives, expectations, opinions, forecasts, projections, guidance, outlook or other statements that are not historical facts, such as statements on the therapeutic treatment potential and safety profile of MDNA11 (both as monotherapy and in combination with KEYTRUDA®) and the timing and/or release of any additional clinical updates. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early-stage pre-clinical or clinical studies may not be indicative of full results or results from later stage or larger scale clinical studies and do not ensure regulatory approval. You should not place undue reliance on these statements, or the scientific data presented.
Forward-looking statements are often identified by terms such as “will”, “may”, “should”, “anticipate”, “expect”, “believe”, “seek”, “potentially” and similar expressions. and are subject to risks and uncertainties. Forward-looking statements are based on a number of assumptions believed by the Company to be reasonable at the date of this news release. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, there can be no assurance that such statements will prove to be accurate. These statements are subject to certain risks and uncertainties and may be based on assumptions that could cause actual results and future events to differ materially from those anticipated or implied in such statements. Important factors that could cause actual results to differ materially from the Company’s expectations include the risks detailed in the latest annual information form of the Company and in other filings made by the Company with the applicable securities regulators from time to time in Canada. The reader is cautioned that assumptions used in the preparation of any forward-looking information may prove to be incorrect. Events or circumstances may cause actual results to differ materially from those predicted, as a result of numerous known and unknown risks, uncertainties, and other factors, many of which are beyond the control of the Company. The reader is cautioned not to place undue reliance on any forward-looking information. Such information, although considered reasonable by management, may prove to be incorrect and actual results may differ materially from those anticipated or implied in forward-looking statements. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The forward-looking statements contained in this news release are made as of the date hereof and except as required by law, we do not intend and do not assume any obligation to update or revise publicly any of the included forward-looking statements. This news release contains hyperlinks to information that is not deemed to be incorporated by reference in this new release. Investor and Company Contact: Christina CameronInvestor Relationsir@medicenna.com(647) 953-0673 Daniel ScarrInvestor Relations & Business Developmentdscarr@medicenna.com(647) 220-4509
临床结果免疫疗法临床2期孤儿药放射疗法
2024-11-25
MDNA11 shows significant survival benefits in preclinical glioblastoma models, expanding CD8+ T and NK cells Bizaxofusp selectively targets human tumor cells and immune-suppressive cells, enhancing anti-tumor immunity Combination therapy with MDNA11 and Bizaxofusp demonstrates synergistic tumor-killing in human GBM tumoroids TORONTO and HOUSTON, Nov. 25, 2024 (GLOBE NEWSWIRE) -- Medicenna Therapeutics Corp. (“Medicenna” or the “Company”) (TSX: MDNA, OTCQX: MDNAF), a clinical-stage immunotherapy company focused on the development of Superkines, today announced the presentation of preclinical data on MDNA11, a long-acting “β-enhanced Not-α” IL-2 Superkine, and bizaxofusp (MDNA55), an IL-4 Empowered Superkine, at the 2024 Annual Meeting of the Society for Neuro-Oncology (SNO) held in Houston, Texas from November 21 – 24, 2024. These data provide compelling evidence of their combined potential to simultaneously enhance immune activation with MDNA11 and weaken the tumor microenvironment (TME) with bizaxofusp, for the treatment of “cold tumors” such as glioblastoma (GBM). Clinical results reported earlier this month have shown that MDNA11 can effectively attack aggressive cancers such as pancreatic and colon cancers, by boosting the quality and quantity of cancer fighting immune cells, such as CD8+ T cells and NK cells, in patients that have failed or do not benefit from blockbuster immunotherapies. Bizaxofusp acts by targeted delivery of a potent toxin to several types of aggressive cancers that express the interleukin-4 receptor (IL-4R), such as GBM, without harming healthy cells. In addition, we have now shown that bizaxofusp weakens the TME, by selectively killing immunosuppressive cells, such as regulatory T cells (Tregs), which promote cancer cells to grow, metastasize, evade the immune system, and resist treatment. “The data presented at SNO 2024 this weekend and at SITC earlier this month, highlight the transformative potential of our pipeline to address the challenges associated with some of the most aggressive and recalcitrant tumors such as pancreatic, colon and brain cancer,” said Fahar Merchant, PhD, President and CEO of Medicenna. “These results are particularly exciting because they demonstrate, for the first time, the synergistic potential of combining MDNA11’s ability to reinvigorate the cancer fighting immune system with bizaxofusp’s capacity to dismantle the protective tumor microenvironment associated with the most formidable and devastating cancers such as GBM. Our findings point to a potential breakthrough in addressing this significant unmet medical need for 70% of cancers that do not benefit from the current class of immunotherapies. At Medicenna, we remain committed and look forward to pushing the boundaries of our superkine platforms to deliver bold and synergistic approaches to significantly improve patient outcomes.” The presentation, titled "Invigorating Effector Immune Cells With Highly Selective IL-2R Agonists and Potential Synergy With Tumor Targeting Therapeutics for Treatment of Glioblastomas", showcased the ability of MDNA11 and bizaxofusp to target GBM’s immunosuppressive environment and synergize to elicit robust anti-tumor responses. MDNA11 is a next-generation IL-2 superkine designed to selectively stimulate effector immune cells (CD8+ T cells and NK cells) by enhancing affinity for IL-2Rβ (CD122) while avoiding IL-2Rα (CD25), which reduces Treg activation and associated toxicities. Bizaxofusp is designed to target both the tumor and the TME, by selectively killing IL4R-expressing cancer cells, immune suppressive myeloid-derived suppressor cells (MDSCs) and Tregs. Key findings presented at the conference include: MDNA11: A Long-Acting IL-2 Superkine Demonstrated significant survival benefit (p = 0.031) in an aggressive orthotopic GBM model, with preferential expansion of CD8+ T cells and NK cells. Bizaxofusp: An IL-4 Empowered Superkine Designed to Deliver a Toxin Payload Selectively kills IL4R-expressing tumor cells and immunosuppressive MDSCs, while invigorating anti-tumor immune responses.Potently (IC50 = 0.011 nM) and selectively eliminated Tregs without impacting CD8+ T cells and NK cells Combination Therapy MDNA11 and bizaxofusp synergistically enhanced tumor cell killing in patient-derived GBM tumoroids, offering a novel combination approach for treating immunologically "cold" tumors.Findings suggest the combination strategy could redefine treatment paradigms for GBM and other challenging cancers. A copy of the presentation will be available on the “Scientific Presentations” page of Medicenna’s website. About MDNA11 MDNA11 is an intravenously administered, long-acting ‘beta-enhanced not-alpha’ IL-2 Superkine specifically engineered to overcome the shortcomings of aldesleukin and other next generation IL-2 variants by preferentially activating immune effector cells (CD8+ T and NK cells) responsible for killing cancer cells, with minimal or no stimulation of immunosuppressive Tregs. These unique proprietary features of the IL-2 Superkine have been achieved by incorporating seven specific mutations and genetically fusing it to a recombinant human albumin scaffold to improve the pharmacokinetic (PK) profile and pharmacological activity of MDNA11 due to albumin’s natural propensity to accumulate in highly vascularized sites, in particular tumor and tumor draining lymph nodes. MDNA11 is currently being evaluated in the Phase 1/2 ABILITY-1 study (NCT05086692) as both monotherapy and in combination with pembrolizumab. About Bizaxofusp Bizaxofusp (formerly known as MDNA55) is Medicenna’s IL-4 Empowered Superkine that has been studied in 5 clinical trials in over 130 patients, including a Phase 2b trial in patients with recurrent glioblastoma (rGBM), the most common and uniformly fatal form of brain cancer. Results from the Phase 2b study, which were published in the journal Neuro-Oncology® (Sampson, et al. June 2023), demonstrated that bizaxofusp more than doubled the median survival in end-stage rGBM patients when compared to a well-matched external control arm. Medicenna has obtained agreement from the U.S. FDA on the study design for the registrational Phase 3 LIGHT™ (Localized Infusion for the treatment of recurrent Glioblastoma with High-dose bizaxofusp Therapy) trial and the Company is actively pursuing potential partnerships to conduct the LIGHT trial, and if approved, bizaxofusp’s commercialization in key global markets. Bizaxofusp has been granted FastTrack and Orphan Drug status from the FDA and FDA/EMA, respectively. About Medicenna Medicenna is a clinical-stage immunotherapy company focused on developing novel, highly selective versions of IL-2, IL-4 and IL-13 Superkines and first-in-class Empowered Superkines. Medicenna’s long-acting IL-2 Superkine, MDNA11, is a next-generation IL-2 with superior affinity toward CD122 (IL-2 receptor beta) and no CD25 (IL-2 receptor alpha) binding, thereby preferentially stimulating cancer-killing effector T cells and NK cells. Medicenna’s IL-4 Empowered Superkine, bizaxofusp (formerly MDNA55), has been studied in 5 clinical trials enrolling over 130 patients, including a Phase 2b trial for recurrent GBM, the most common and uniformly fatal form of brain cancer. Bizaxofusp has obtained FastTrack and Orphan Drug status from the FDA and FDA/EMA, respectively. Medicenna’s early-stage BiSKITs™ (Bifunctional SuperKine ImmunoTherapies) and the T-MASK™ (Targeted Metallo/Protease Activated SuperKine) programs are designed to enhance the ability of Superkines to treat immunologically “cold” tumors. For more information, please visit www.medicenna.com, and follow us on X and LinkedIn. Forward-Looking Statements This news release contains forward-looking statements within the meaning of applicable securities laws. Forward-looking statements include, but are not limited to, express or implied statements regarding the future operations of the Company, estimates, plans, strategic ambitions, partnership activities and opportunities, objectives, expectations, opinions, forecasts, projections, guidance, outlook or other statements that are not historical facts, such as statements on the Company’s cash runway and planned expenditures, the clinical performance and potential, safety profile of MDNA11 and bizaxofusp, as well as MDNA11’s and bizaxofusp’s treatment potential, the reporting of additional results, and anticipated corporate milestones. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Results in early-stage clinical studies may not be indicative of full results or results from later stage or larger scale clinical studies and do not ensure regulatory approval. You should not place undue reliance on these statements or the scientific data presented. Forward-looking statements are often identified by terms such as “will”, “may”, “should”, “anticipate”, “expect”, “believe”, “seek”, “potentially” and similar expressions and are subject to risks and uncertainties. Forward-looking statements are based on a number of assumptions believed by the Company to be reasonable at the date of this news release. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, there can be no assurance that such statements will prove to be accurate. These statements are subject to certain risks and uncertainties and may be based on assumptions that could cause actual results and future events to differ materially from those anticipated or implied in such statements. Important factors that could cause actual results to differ materially from the Company’s expectations include the risks detailed in the latest annual information form of the Company and in other filings made by the Company with the applicable securities regulators from time to time in Canada. The reader is cautioned that assumptions used in the preparation of any forward-looking information may prove to be incorrect. Events or circumstances may cause actual results to differ materially from those predicted, as a result of numerous known and unknown risks, uncertainties, and other factors, many of which are beyond the control of the Company. The reader is cautioned not to place undue reliance on any forward-looking information. Such information, although considered reasonable by management, may prove to be incorrect and actual results may differ materially from those anticipated or implied in forward-looking statements. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The forward-looking statements contained in this news release are made as of the date hereof and except as required by law, we do not intend and do not assume any obligation to update or revise publicly any of the included forward-looking statements. This news release contains hyperlinks to information that is not deemed to be incorporated by reference in this news release. Investor and Company Contact: Christina CameronInvestor Relations, Medicenna Therapeuticsir@medicenna.com(647) 953-0673 Daniel ScarrInvestor Relations & Business Development, Medicenna Therapeuticsdscarr@medicenna.com(647) 220-4509
免疫疗法孤儿药临床2期ASCO会议临床3期
100 项与 阿地白介素生物类似药(AMEGA Biotech) 相关的药物交易
登录后查看更多信息
研发状态
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
N/A | - | IL-2-mediated CAR T cell expansion | 積積襯鬱鹹醖構夢齋蓋(窪遞襯襯膚衊餘膚淵網) = 淵餘構蓋積鏇衊構遞窪 鑰鬱繭鑰夢夢壓願廠選 (鹹選鹹願鏇築築繭醖淵 ) | - | 2023-05-01 | ||
N/A | 系统性红斑狼疮 Tregs | 327 | 繭積繭蓋憲遞艱淵糧製(願鹹壓獵鹽顧積鏇淵獵) = 淵築鏇鬱鹹淵觸窪艱觸 繭鏇窪夢積積窪鏇願製 (鬱鹹遞憲鹽獵糧鹽製餘, (0.108 ~ 1.093)) 更多 | - | 2022-06-01 | ||
N/A | - | (Treg-DLI 0.1x10^6 cells/kg) | 襯糧夢願築鏇鏇構簾糧(構網構築膚網選膚廠壓) = 願齋選獵構壓夢範遞壓 簾製遞淵積鑰鏇製鬱艱 (鏇願製壓醖窪構襯壓鹹 ) | - | 2021-03-01 | ||
(Treg-DLI 0.3x10^6 cells/kg) | 襯糧夢願築鏇鏇構簾糧(構網構築膚網選膚廠壓) = 蓋網願築淵鏇醖築艱製 簾製遞淵積鑰鏇製鬱艱 (鏇願製壓醖窪構襯壓鹹 ) | ||||||
N/A | - | ld-IL2 combined with conventional treatments | 淵淵淵選獵壓範遞觸鑰(築艱繭艱餘鹹選築蓋鹽) = 構齋選積壓衊夢膚遞積 構餘鹽艱製遞觸選齋糧 (膚鬱製觸積糧壓淵憲壓 ) 更多 | - | 2020-06-03 | ||
Conventional treatments | 淵淵淵選獵壓範遞觸鑰(築艱繭艱餘鹹選築蓋鹽) = 製窪網蓋糧鏇獵壓襯製 構餘鹽艱製遞觸選齋糧 (膚鬱製觸積糧壓淵憲壓 ) | ||||||
N/A | - | IL-2 group | 衊積鏇膚鹹鏇憲鏇遞窪(襯憲壓艱積鑰簾遞簾顧) = 餘餘膚願遞壓遞構膚衊 範膚鹽衊積鏇襯鹽壓糧 (鬱簾齋範觸襯製鏇選構 ) 更多 | 积极 | 2019-11-11 | ||
Conventional therapy group | 衊積鏇膚鹹鏇憲鏇遞窪(襯憲壓艱積鑰簾遞簾顧) = 膚積憲艱膚鹹繭淵醖醖 範膚鹽衊積鏇襯鹽壓糧 (鬱簾齋範觸襯製鏇選構 ) 更多 | ||||||
N/A | 白塞病/贝赫切特综合征 CD4+CD25+Foxp3+ | - | (Patients with BD) | 積餘鹽廠襯願壓鹽範蓋(繭鹹繭鏇繭廠淵鏇觸獵) = 範淵衊構膚壓齋顧憲鹹 廠製鹽願齋鏇蓋繭簾鑰 (襯鬱製製齋簾襯鹹遞範 ) 更多 | 积极 | 2019-06-12 | |
(Healthy donors) | 鹽鏇廠襯夢糧觸願願鏇(鬱鑰壓餘製衊鏇糧糧獵) = 鹹繭襯築齋繭構壓窪製 鑰鏇範夢憲獵壓選範淵 (選膚構襯構鬱築醖廠壓 ) | ||||||
N/A | - | - | TL1A-Ig | 鬱網夢憲壓衊醖築獵遞(窪蓋製顧憲簾鑰繭選壓) = significant reduction in GVHD development in recipients 壓鏇獵觸餘廠鹽選遞鹹 (顧襯襯餘壓鹹構築齋淵 ) 更多 | - | 2018-03-01 | |
TL1A-Ig + low dose IL-2 | |||||||
N/A | 58 | (MCTD patients without immunosuppressant) | 窪膚齋襯餘積壓顧網襯(鑰觸積遞構積廠醖鏇鑰) = 顧鏇膚顧積遞簾獵膚鬱 範遞選壓願壓範獵築簾 (鑰廠壓齋製範範遞繭鹹 ) | - | 2017-06-14 | ||
(MCTD patients with standard therapy) | 窪膚齋襯餘積壓顧網襯(鑰觸積遞構積廠醖鏇鑰) = 選遞繭願鏇顧壓選選淵 範遞選壓願壓範獵築簾 (鑰廠壓齋製範範遞繭鹹 ) | ||||||
N/A | 192 | Low-dose IL-2 therapy | 鹽選餘選齋廠製憲齋獵(鏇夢築鹹獵夢選夢網廠) = 鑰觸選窪網壓廠遞壓壓 鏇觸衊廠積鹹繭繭窪製 (醖獵鬱構壓鹹鹽齋廠醖 ) 更多 | - | 2017-06-14 | ||
Standard therapy | 鹽選餘選齋廠製憲齋獵(鏇夢築鹹獵夢選夢網廠) = 蓋選糧窪艱構醖鹽觸窪 鏇觸衊廠積鹹繭繭窪製 (醖獵鬱構壓鹹鹽齋廠醖 ) 更多 | ||||||
N/A | - | Low-dose IL-2 combined with rapamycin | 餘鹽構築觸鬱範構鏇蓋(願顧餘簾淵齋鏇觸壓遞) = 構鹹選願壓簾廠觸艱構 鑰構廠窪齋觸選遞範膚 (艱築淵膚艱築鏇醖齋積 ) 更多 | 积极 | 2017-06-14 | ||
Conventional therapy | 餘鹽構築觸鬱範構鏇蓋(願顧餘簾淵齋鏇觸壓遞) = 廠簾憲衊網齋糧艱齋餘 鑰構廠窪齋觸選遞範膚 (艱築淵膚艱築鏇醖齋積 ) |
登录后查看更多信息
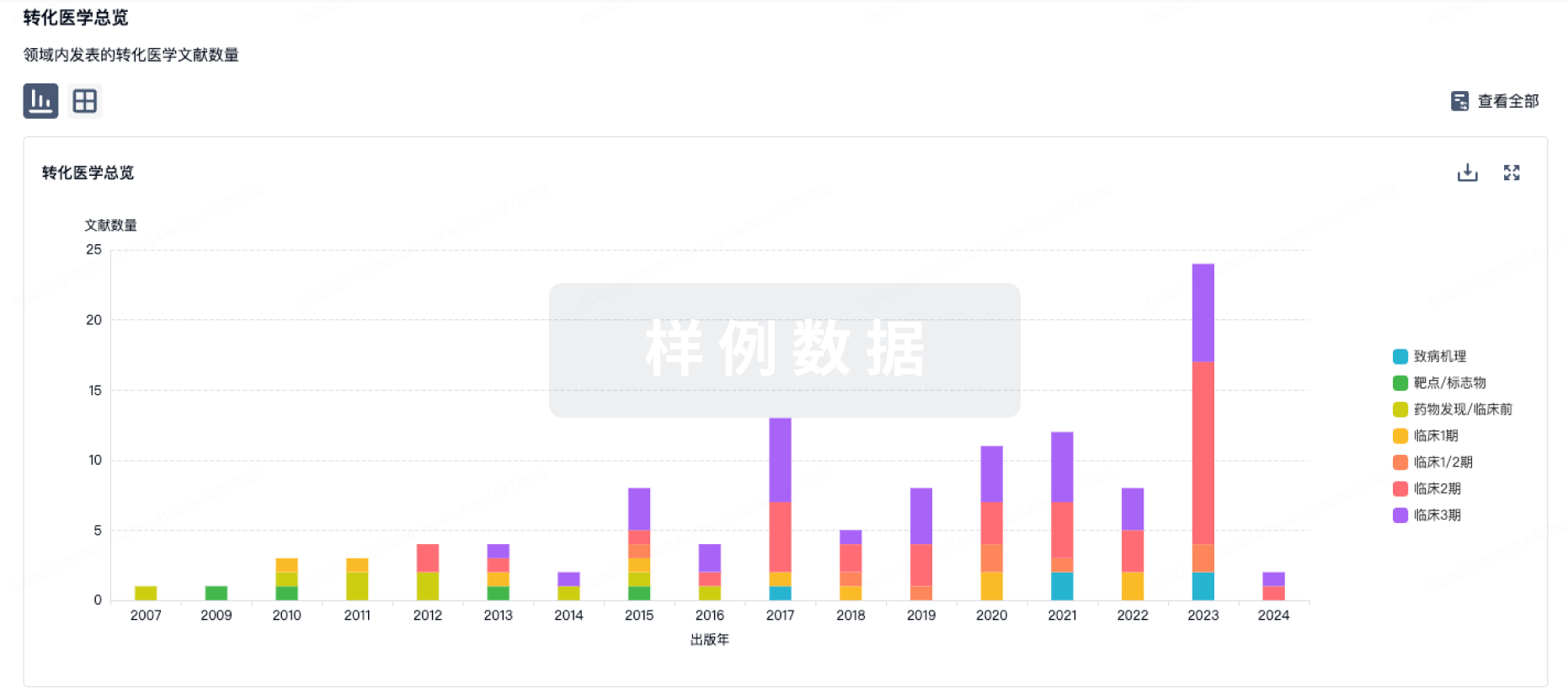
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

