预约演示
更新于:2025-05-07
Facial Hypertrichosis
面部多毛症
更新于:2025-05-07
基本信息
别名 FACIAL HYPERTRICHOSIS、Facial Hypertrichosis、Facial hypertrichosis + [2] |
简介 Excessive, increased hair growth located in the facial region. [] |
关联
1
项与 面部多毛症 相关的药物2
项与 面部多毛症 相关的临床试验TCTR20220205004
Efficacy and Safety of Topical 3% Minoxidil for Facial Hair Enhancement in Transmen: A Randomized, Double-blind, Placebo-controlled Trial
开始日期2022-02-01 |
申办/合作机构- |
EUCTR2005-003537-41-DE
Efficacy of the treatment with VANIQA 11,5% creme in patients suffering from hirsutism of the upper lip - Treatment with VANIQA
开始日期2005-10-27 |
申办/合作机构 |
100 项与 面部多毛症 相关的临床结果
登录后查看更多信息
100 项与 面部多毛症 相关的转化医学
登录后查看更多信息
0 项与 面部多毛症 相关的专利(医药)
登录后查看更多信息
72
项与 面部多毛症 相关的文献(医药)2025-12-31·Journal of Dermatological Treatment
Low-dose oral minoxidil in a case of short anagen syndrome
Article
作者: Ungar, Benjamin ; Morsia, Serena ; Sharma, Divija
2024-12-01·Orthodontics & Craniofacial Research
In utero nicotine exposure affects murine palate development
Article
作者: Durham, Emily L. ; Mohi, Amr ; Boyce, Mark ; Cray, James J. ; Vyas, Heema
2024-01-01·Journal of Clinical Sleep Medicine
Surgical correction of neonatal obstructive sleep apnea due to a temporomandibular joint ankylosis
作者: Pesis, Michael ; Givol, Navot ; Goldbart, Aviv
分析
对领域进行一次全面的分析。
登录
或
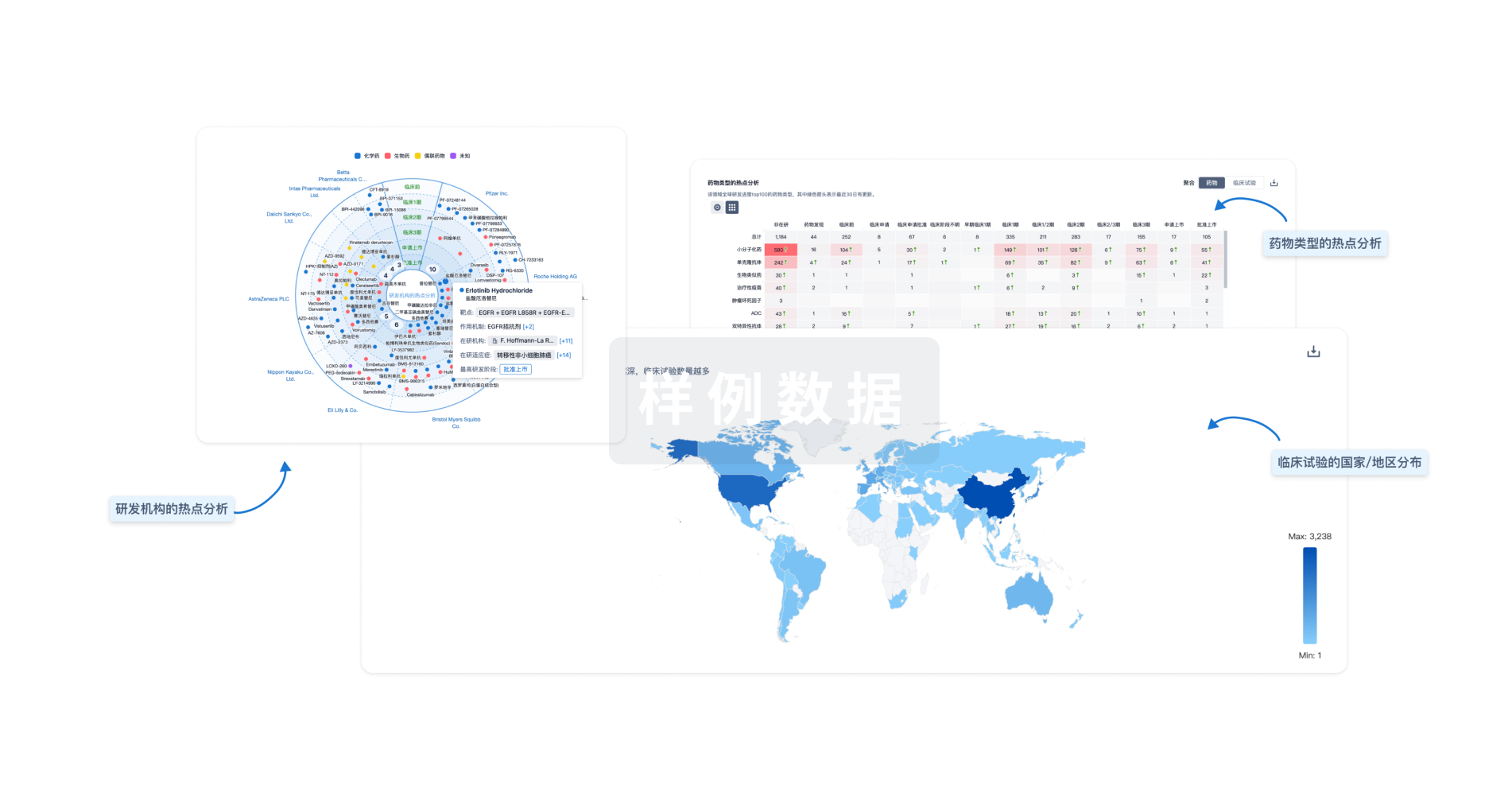
Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

