预约演示
更新于:2025-01-23
Infection pyogenic
化脓性感染
更新于:2025-01-23
基本信息
别名 Infection pyogenic、Pyogenic Infection、infection pyogenic + [4] |
简介 An infection caused by pus-producing organisms. |
关联
16
项与 化脓性感染 相关的临床试验CTR20242085
头孢泊肟酯片在空腹及餐后条件下的人体生物等效性试验
研究健康受试者在空腹及餐后条件下,单次口服由浙江巨泰药业有限公司生产的头孢泊肟酯片(受试制剂T,规格:100mg)与相同条件下单次口服由Daiichi Sankyo Co., Ltd.持证的头孢泊肟酯片(参比制剂R,商品名:BANAN®,规格:100mg)的药动学特征,评价两制剂的生物等效性及安全性。
开始日期2024-06-24 |
申办/合作机构 |
CTR20241822
头孢地尼颗粒在健康受试者中单中心、随机、开放、两制剂、单剂量、双周期、双交叉空腹和餐后状态下的生物等效性试验
本研究考察健康受试者在空腹及餐后条件下,单次口服由康普药业股份有限公司生产的头孢地尼颗粒(受试制剂,规格:50mg)与相同条件下单次口服由LTLファーマ株式会社持证的头孢地尼细粒剂(参比制剂,商品名:Cefzon®,规格:50mg/0.5g)的药动学特征,评价两制剂间的生物等效性及安全性,为该受试制剂的注册申请提供依据。
开始日期2024-05-18 |
申办/合作机构 |
CTRI/2023/10/058448
A study to determine the aetiology of Infectious Spondylodiscitis and todevelop a diagnostic scoring system - NIL
开始日期2023-10-13 |
申办/合作机构- |
100 项与 化脓性感染 相关的临床结果
登录后查看更多信息
100 项与 化脓性感染 相关的转化医学
登录后查看更多信息
0 项与 化脓性感染 相关的专利(医药)
登录后查看更多信息
1,215
项与 化脓性感染 相关的文献(医药)2024-12-02·Cureus
Cervical Spinal Epidural Abscess From Haemophilus influenzae in an Adult: A Case Report
Article
作者: Jung, Julianna ; Qiao, Luxi ; Choi, April
2024-12-01·Acta Paediatrica
Role of childhood immunisation coverage in the increase of pyogenic infection in children after pandemic
Letter
作者: Trombetta, Andrea
2024-12-01·Acta Paediatrica
Increase of pyogenic bacterial infections after relaxation of social distancing measures linked to COVID ‐19 pandemic
Article
作者: Minute, Marta ; Rossetti, Vanessa ; Vanz, Daniele ; Boscarelli, Alessandro ; Cozzi, Giorgio ; Amaddeo, Alessandro
12
项与 化脓性感染 相关的新闻(医药)2025-01-14
EAST HARTFORD, Conn., Jan. 14, 2025 /PRNewswire/ -- icotec is proud to announce that it has received FDA clearance for the use of BlackArmor® implants in the treatment of de novo spinal infections.
icotec is honored to be the first and only company in the United States with FDA 510(k) clearance for stabilizing the spine in de novo spinal infections, including discitis, osteomyelitis, pyogenic infection of the intervertebral disc, and other spondylopathies. "Over 15,000 patients receive spinal stabilizations due to an infection in the spine in the USA every year," says Chris Eigenmann, CEO icotec Medical US, "being able to help these patients with an implant that allows for improved post-operative monitoring and visualization is a great opportunity and privilege." In addition to the on-label designation, the FDA has also granted a Breakthrough Device Designation (BDD) for this indication across the entirety of icotec's BlackArmor® spinal stabilization portfolio in recognition of the significant unmet need and the novel benefits that BlackArmor® technology may offer these patients.
Furthermore, following the FDA decision, Centers for Medicare and Medicaid Services (CMS) approved BlackArmor® for New Technology Add-on Payment (NTAP), which is awarded when innovative medical technologies are determined to significantly improve the diagnosis or treatment of Medicare beneficiaries.
Clinical Value and Research
icotec's BlackArmor® implants offer reduced artifacts from radiolucent Carbon/PEEK material, which enables improved imaging in the post-operative setting as well as in the monitoring of infection.
Supporting clinical studies, such as Burkhardt et al. (2021), have demonstrated safety, with equivalent complication rates to titanium implants, and showed the benefit of reduced imaging artifact, which provides additional diagnostic information for patients stabilized with Carbon/PEEK implants. "Thanks to the clinical data that was gathered in Germany over the past several years," explains Roger Stadler, Group CEO, "we can now offer a proven, dedicated implant option to spinal infection patients in the United States. We feel honored that the FDA has recognized the potential of carbon fiber implants for this patient population and granted a Breakthrough Device Designation based on the clinical data available." Additional research to support the efficacy and safety of BlackArmor® implants in treating spinal infections is ongoing.
Breakthrough Device Designation (BDD) and New Technology Add-On Payment (NTAP)
As of October 1, 2024, hospitals are eligible to receive additional payments for the VADER® pedicle screw system of up to $28,000 for Medicare Fee-for-Service patients. This NTAP designation is a testament to the innovative nature and clinical value of icotec's products, and its commitment to advancing the standard of care for patients with spinal infections.
About icotec
icotec is the leading company for the treatment of spinal tumors and spinal infections with a new generation of high-tech implants. With its BlackArmor® Carbon/PEEK implants, icotec combines cutting-edge technologies and industry expertise to deliver innovative and reliable solutions for spine surgeons and their patients and is dedicated to advancing the field of spinal implantation. With a track record of clinical success and a commitment to continuous innovation, icotec is poised to shape the future of spinal surgery. The comprehensive product portfolio has received FDA clearance and is supported by numerous Key Opinion Leaders and Cancer Therapy Centers worldwide. More information can be found at
.
For more information or questions regarding this new indication, please contact John Clough, Vice President of Global Marketing and New Indications.
[email protected]
Logo -
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE.COM?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
上市批准
2024-12-17
来源:国家药监局 编辑:wangxinglai2004
2024年12月17日,国家药监局发布了非处方药转换公告,涉及品种为酮洛芬贴片,要求持有人于2025年09月13日前,完成修订说明书事项向省级药品监督管理部门备案。
公告主要内容
根据《处方药与非处方药分类管理办法(试行)》(原国家药品监督管理局令第10号)的规定,经国家药品监督管理局组织论证和审核,酮洛芬贴片由处方药转换为非处方药。品种名单(见附件1)及其非处方药说明书范本(见附件2)一并发布。
请相关药品上市许可持有人于2025年9月13日前,依据《药品注册管理办法》(国家市场监督管理总局令第27号)等有关规定,向省级药品监督管理部门提交修订说明书备案,并将说明书修订内容及时通知相关医疗机构、药品经营企业等单位。
非处方药说明书范本规定内容之外的说明书其他内容,按原批准证明文件执行。药品标签涉及相关内容的,应当一并修订。药品上市许可持有人提交备案之日起生产的药品,不得继续使用原药品说明书。
非处方药说明书范本
酮洛芬贴片说明书
请仔细阅读说明书并按说明使用或在药师指导下购买和使用
[药品名称]
通用名称:酮洛芬贴片
商品名称:
英文名称:
汉语拼音:
[成份]
[性状]
[作用类别]本品为镇痛类非处方药药品。
[适应症]主要用于骨关节炎的症状缓解。
[规格]每片7厘米×10厘米,含酮洛芬20毫克
[用法用量]局部外用,除去防粘纸,贴敷于患处。一日一次,每日用量不超过8贴。
[不良反应]
1.偶有用药部位发生散在皮疹、皮肤潮红、瘙痒等不良反应,大剂量应用时,可能出现胃部刺激症状。文献中有老年人长期大量使用出现溃疡性肠出血的个案报道。
2.与酮洛芬贴片活性成份和用药途径相同的酮洛芬凝胶主要不良反应表现:偶尔出现接触性皮炎(如皮肤发红、皮疹、瘙痒、水疱、糜烂、刺激感、肿胀等)、皮肤干燥及色素沉着。直射光(紫外线)照射后可出现光过敏反应,皮疹可扩散到全身。偶尔出现过敏性反应(如荨麻疹、呼吸困难、颜面浮肿等)。
[禁忌]
1.对本制剂中的药物成分有既往过敏史者禁用。
2.对其他非甾体抗炎药过敏者禁用。
3.对使用阿司匹林或其它前列腺素合成酶抑制剂引起哮喘、荨麻疹或急性鼻炎的患者禁用。
4.破损皮肤或患处有化脓性感染者禁用。
5.眼的黏膜处禁用。
6.胃、十二指肠溃疡出血患者禁用。
7.有光敏反应史者(暴露于阳光下后出现皮肤反应)禁用。
8.有紫外线防晒剂或者香氛过敏史者禁用。
[注意事项]
1.避免接触眼睛和其他黏膜(如口、鼻等)。
2.不得用于皮肤破损处及感染性创口。
3.孕妇及哺乳期妇女不宜使用。
4.老人及小儿应慎用,如需使用,用药前应咨询医师并由医师指导。
5.支气管哮喘患者慎用(可引起哮喘发作),用药后如出现干罗音、喘鸣、呼吸困难等初期症状时,应停药,及时去医院就诊。
6.交叉过敏:对阿司匹林或其他非甾体抗炎药过敏者,本品可有交叉过敏反应。对阿司匹林过敏的哮喘患者,本品也可引起支气管痉挛。
7.胃及十二指肠溃疡患者慎用。
8.心脏、肾脏或肝脏功能受损的患者应慎用本品。
9.用抗炎镇痛药治疗不是病因治疗,而是对症治疗。
10.用药部位如有烧灼感、瘙痒、红肿等皮肤反应,应停药,并将局部药物洗净,必要时向医师咨询。
11.在整个治疗期间和治疗结束后的2周内,用合适的衣物保护治疗区域免受日光照射,以防止任何光敏反应的风险。
12.治疗7天后,不见好转,应咨询医师。
13.对本品过敏者禁用,过敏体质者慎用。
14.本品性状发生改变时禁止使用。
15.请将本品放在儿童不能接触的地方。
16.儿童必须在成人监护下使用。
17.如正在使用其他药品,使用本品前请咨询医师或药师。
[药物相互作用]
如与其他药物同时使用可能会发生药物相互作用,详情请咨询医师或药师。
[药理作用]本品为外用芳基丙酸类非甾体抗炎药,具有镇痛、抗炎作用,其机理可能主要是抑制前列腺素的合成。
[贮藏]
[包装]
[有效期]
[执行标准]
[批准文号]
[说明书修订日期]
[上市许可持有人]
名称:
注册地址:
邮政编码:
电话号码:
传真号码:
网址:
[生产企业]
企业名称:
生产地址:
邮政编码:
电话号码:
传真号码:
网址:
如有问题可与药品上市许可持有人联系
(注:本说明书范本原则上不得删减,如原批准说明书的安全性内容较本范本内容更全面或更严格的,应保留原批准内容。)
2024-07-13
·梅斯医学
据央视新闻消息,日本媒体12日报道,俗称“食人菌”感染症的链球菌中毒休克综合征患者数量在日本持续增加。截至6月30日,日本今年已确认1144例“食人菌”感染症病例。这一数字较去年同期出现了显著增长,且已逼近并超越了去年全年941例的历史高点。这一系列数据不仅揭示了该病症在日本的严峻形势,也向全球公共卫生安全敲响了警钟。
另据日本妇产科医师协会发布的一项调查,从去年7月到今年3月,日本已有5名孕产妇因此死亡。
链球菌中毒休克综合征是一种由溶血性链球菌引发的败血性休克,感染途径一般为经鼻腔、咽喉黏膜的飞沫传播和经伤口等的接触传播。患者从出现症状到发展为休克和多脏器衰竭只需要24至48小时,且致死率高达30%,因此其致病菌在日本被称为“食人菌”。
据北京佑安医院感染科主任医师李侗曾介绍,这种疾病并非某一个地区特有,美国等国家也报道过,比较高的年份每年发生600例至800例,只是日本今年比较多。
为何出现病例的增长还没有明确的结论。一种看法是跟病原体周期性流行有关,类似支原体也有大年小年;还有认为新冠疫情之后人群活动增加导致,如旅游、聚集等。
根据日本披露的数据,今年检测出比较多的M1UK谱系菌株,该菌株毒性和传播力更强。
流行病学
1、传染源
传染源主要为上呼吸道感染患者和人畜化脓性感染部位。
2、传播途径
经鼻腔、咽喉黏膜的飞沫传播;经伤口等的接触传播。
3、易感人群
(1)老年人:与咽炎等普通溶血性链球菌感染症易感染儿童不同,“食人菌”感染症多发于30岁以上的成年人。
(2)有伤口或皮肤溃破的人:患有溃疡性皮肤疾病的人、最近接受过手术的人、最近感染过带状疱疹和水痘等容易导致皮肤溃烂的疾病的人、有糖尿病等基础疾病的人、常用止痛片或非甾体抗炎药的人。
临床表现
链球菌中毒休克综合征的临床表现多种多样,但通常在感染后24至48小时内迅速恶化,以下是一些主要症状:
1、高热
患者通常会出现突然的高热,体温可达39℃以上。这种高热常伴有寒战和全身不适。
2、皮疹
许多患者会出现弥漫性红斑样皮疹,类似于晒伤。这种皮疹通常分布在四肢和躯干,并可能发展为剥脱性皮炎。
3、血压下降
由于全身性的血管扩张,患者的血压会迅速下降,导致休克。患者常出现头晕、乏力、甚至意识丧失等症状。
4、器官功能障碍
随着病情的进展,患者可能会出现肾功能衰竭、肝功能障碍、呼吸衰竭和心力衰竭等多脏器功能衰竭的症状。这些症状的出现通常预示着病情的进一步恶化,需紧急医疗干预。
诊断依据
1、从可疑食品中经培养分离出乙型溶血性链球菌。
2、从2个或2个以上咽拭、漱口液中检出乙型溶血性链球菌。
3、患者恢复期血清抗体滴度呈4倍增长有诊断意义。
治疗
对A族β-溶血性链球菌感染,现在的抗生素治疗是有效的,青霉素、阿莫西林、头孢类抗生素的疗效都较好。对于链球菌中毒休克综合征,在使用抗生素的同时,要根据具体情况纠正酸中毒、改善微循环等。
预防和控制
1、做好个人卫生,勤洗手、注意室内通风换气,流行期间注意戴口罩,避免前往人员密集、通风不良的公共场所。
2、防止带菌人群对各种食物的污染,患局部化脓性感染、上呼吸道感染的人员要暂停与食品接触的工作。
3、防止对牛奶及其制品的污染,牛奶场要定期对生产中的奶牛进行体检,坚持挤奶前消毒,一日发现患化脓性乳腺炎的奶牛要立即隔离,奶制品要用消毒过的原料,并注意低温保存。
4、在动物屠宰过程中,应严格执行检验法规,割除病灶并以流水冲洗;在肉制品加工过程中发现化脓性病灶应整块剔除。
撰文 | 梅斯医学
编辑 | Swagpp
● 太重磅!正式迈入5+3+x时代!上海发布专培政策,专培证优先晋升高级职称、专培合格可授予博士学位!10年时间,终于从试行到确立!
● 警惕厨房常见的清洁海绵!南大、东南团队研究:磨损后每克释放出650万个塑料微粒,恐伤脑伤肝
● 震撼!资深医生吐槽:现在的年轻医生为啥不好带了?不懂还不去学,脾气又大,受点委屈就离职!网友:年轻人自我意识觉醒,这是时代进步!
版权说明:梅斯医学(MedSci)是国内领先的医学科研与学术服务平台,致力于医疗质量的改进,为临床实践提供智慧、精准的决策支持,让医生与患者受益。欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。
点击下方「阅读原文」 立刻下载梅斯医学APP!
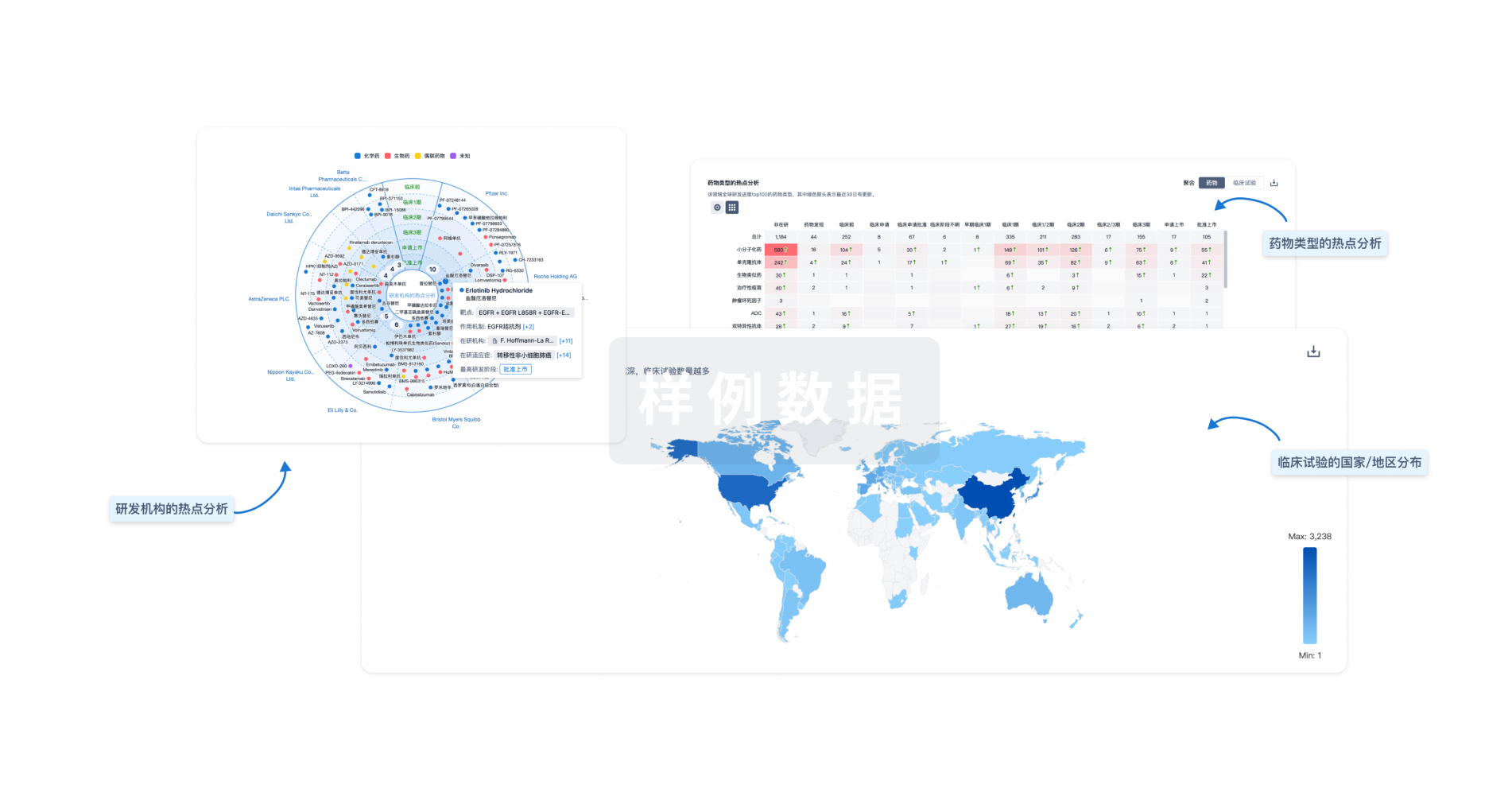
分析
对领域进行一次全面的分析。
登录
或
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

