预约演示
更新于:2025-05-07
Enterococcal Infection
肠球菌感染
更新于:2025-05-07
基本信息
别名 Enterococcal infection、Enterococcal infection NOS、Enterococcal infections + [7] |
简介- |
关联
10
项与 肠球菌感染 相关的药物靶点- |
作用机制 细胞膜调节剂 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
靶点- |
作用机制 细胞膜调节剂 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
靶点- |
作用机制 细胞膜调节剂 |
非在研适应症- |
最高研发阶段临床前 |
首次获批国家/地区- |
首次获批日期1800-01-20 |
4
项与 肠球菌感染 相关的临床试验NCT05394298
Randomized Non-inferiority Clinical Trial to Evaluate the Effectiveness and Security of Therapy for Non Complicated Enterococcal Bacteremia.
Randomized clinical trial to determine the optimal duration of antibiotic treatment for E. Faecalis or E. faecium bacteraemia, following an innovative DOOR / RADAR (Desirability of Outcome Ranking (DOOR) and Response Adjusted for Duration of Antibiotic Risk (RADAR)) analysis methodology.
Phase IV clinical trial, open-labelled, randomized, pragmatic, multicenter study to demonstrate non-inferiority of a 7-day antibiotic regimen vs. 14 days in the treatment of bacteremia due to E. faecalis or E. faecium.
Phase IV clinical trial, open-labelled, randomized, pragmatic, multicenter study to demonstrate non-inferiority of a 7-day antibiotic regimen vs. 14 days in the treatment of bacteremia due to E. faecalis or E. faecium.
开始日期2022-07-11 |
申办/合作机构- |
NCT04777240
Characterization of Enterococci; Distribution of Virulence Markers, Virulence Genes and Antibiotic Resistance Pattern of the Isolated Species
Study on Characterization of Enterococci because nowadays it become an important cause of nosocomial infections .detection of the most common two species of Enterococci and most common virulence factors & its genes with determination of antibiotics sensitivity test for the isolated strains
开始日期2020-02-01 |
申办/合作机构 |
NCT04410276
VENOUS: A Translational Study of Enterococcal Bacteremia
The purpose of this study is to assemble a multicenter prospective cohort of patients with enterococcal bloodstream infections (BSIs) to provide data on outcomes of patients with enterococcal BSIs for sample size calculations for future trials, as well as to characterize enterococcal isolates causing BSIs in order to comprehensively dissect the molecular epidemiology of infecting organisms for future studies.
开始日期2016-08-01 |
100 项与 肠球菌感染 相关的临床结果
登录后查看更多信息
100 项与 肠球菌感染 相关的转化医学
登录后查看更多信息
0 项与 肠球菌感染 相关的专利(医药)
登录后查看更多信息
1,044
项与 肠球菌感染 相关的文献(医药)2025-05-01·Infection Control & Hospital Epidemiology
Does PCR-based pathogen identification reduce mortality in bloodstream infections? Insights from a difference-in-difference analysis
Article
作者: Gago, Juan ; Thorpe, Lorna E ; Torres, Victor J ; Takats, Courtney ; Shopsin, Bo ; Renson, Audrey
2025-04-01·British Journal of Clinical Pharmacology
Systematic review and meta‐analysis of vancomycin therapeutic level for treatment of vancomycin‐sensitive enterococcal infections
Review
作者: Katip, Wasan ; Kasatpibal, Nongyao ; Rayanakorn, Ajaree ; Lee, Shaun Wen Huey
2025-03-04·Microbiology Spectrum
Role of sortase-assembled Ebp pili in
Enterococcus faecalis
adhesion to iron oxides and its impact on extracellular electron transfer
Article
作者: Lam, Ling Ning ; Ajo-Franklin, Caroline M. ; Heras, Begoña ; Tolar, Joe ; Watts, Thomas Dean ; Ho, Foo Kiong ; Matysik, Artur ; Low, Pui Man ; Paxman, Jason J. ; Chua, Zhi Sheng ; Marsili, Enrico ; Wong, Jun Jie ; Choo, Pei Yi ; Kline, Kimberly A. ; Chong, Kelvin Kian Long
10
项与 肠球菌感染 相关的新闻(医药)2025-03-20
Received European Commission approval for GOHIBIC® (vilobelimab) for the treatment of SARS-CoV-2-induced acute respiratory distress syndrome (ARDS)Achieved 30-patient recruitment milestone in Phase 3 vilobelimab trial in pyoderma gangrenosum (PG) to enable an expected interim analysis for trial size adaptation or futility by the end of May 2025Dosed first patient in Phase 2a trial for oral C5aR inhibitor, INF904, with topline data in chronic spontaneous urticaria (CSU) and hidradenitis suppurativa (HS) expected in summer 2025Multiple data presentations at AAD 2025 highlighting the potential of vilobelimab in reducing systemic inflammationCash, cash equivalents and marketable securities of €55.2 million as of December 31, 2024Additional €28.7 million ($30.0 million) in gross proceeds subsequently raised by an underwritten public offering of ordinary shares and pre-funded warrants on February 18, 2025InflaRx’s cash runway significantly extended, with sufficient cash, cash equivalents and marketable securities to fund currently planned operations into 2027 JENA, Germany, March 20, 2025 (GLOBE NEWSWIRE) -- InflaRx N.V. (Nasdaq: IFRX), a biopharmaceutical company pioneering anti-inflammatory therapeutics by targeting the complement system, today announced its financial results for the year ended December 31, 2024, highlighting recent operational achievements and expected milestones for 2025. Prof. Niels C. Riedemann, Chief Executive Officer and Founder of InflaRx, commented: “2024 was a highly productive year for InflaRx, with the company achieving all major development and regulatory goals across its pipeline programs and making meaningful progress in addressing critical medical needs in inflammatory diseases.” He continued: “We look forward to a catalyst-rich year in 2025 as we continue to advance our pipeline, including reaching the interim analysis for the Phase 3 trial with vilobelimab in pyoderma gangrenosum and reporting top-line Phase 2a data in chronic spontaneous urticaria and hidradenitis suppurativa with INF904, our oral C5aR inhibitor with best-in-class potential." Select recent highlights and expected milestones INF904 in CSU and HS – Topline Phase 2a data expected in summer 2025 In December 2024, InflaRx announced that the first patient had been dosed in its Phase 2a basket study with INF904 in CSU and HS. This is a multi-center, open-label study evaluating multiple INF904 dosing regimens over 4 weeks of treatment in a total of 75 patients (45 in CSU and 30 in HS). The goal of the trial is to generate additional safety and pharmacokinetic (PK) data and to provide signs of clinical benefit. After the 4-week treatment period, patients will be followed for an additional 4 weeks. Topline data from this study are expected in the summer of 2025, with a goal of informing the planning and design of a larger, longer-term Phase 2b study by year-end 2025. InflaRx believes CSU and HS each has potential addressable markets of $1 billion or more for INF904. The Company also believes INF904 could address meaningful opportunities in additional immuno-dermatology and immuno-inflammatory indications, including in nephrology, neurology and hematology. While InflaRx intends to focus its resources on its immediate goals addressing CSU and HS, the Company continues to assess and monitor the value of pursuing additional areas and applications via potential future collaborations with partners. Vilobelimab in PG – Pivotal Phase 3 trial interim analysis expected by the end of May 2025 In November 2024, InflaRx announced that it had achieved the 30-patient recruitment milestone in its ongoing Phase 3 vilobelimab trial in PG. This is expected to enable the interim analysis for trial size adaptation or futility by the end of May 2025. Trial enrollment continues. The study dosed its first patient in November 2023 and has an adaptive design with an interim analysis (unblinded only for the independent data monitoring committee), which is planned when 30 patients randomized 1:1 to the two arms have completed treatment. The interim analysis with a set of predefined rules will consider the then-observed difference in complete target ulcer closure between the two arms and will then determine whether the trial sample size will be adapted or whether the trial should be stopped due to futility. The enrollment period is projected to last at least two years, and its overall period will depend on the total trial size after sample size adaptation. The Phase 3 trial is a multi-national, randomized, double-blind, placebo-controlled pivotal study assessing the benefit of vilobelimab for treating ulcerative PG, a rare, chronic inflammatory form of neutrophilic dermatosis characterized by accumulation of neutrophils in the affected skin areas. The trial has two arms: (1) vilobelimab plus a low dose of corticosteroids and (2) placebo plus the same dose of corticosteroids, both tapered over an 8-week period. The primary endpoint of the study is complete closure of the target ulcer measured at two consecutive visits at any time up to 26 weeks after initiation of treatment. Vilobelimab has been granted orphan drug designation for the treatment of PG by both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), as well as fast track designation by the FDA. Vilobelimab presentations at the 2025 AAD Annual Meeting During the 2025 American Academy of Dermatology (AAD) Annual Meeting held March 7 – 11 in Orlando, FL, vilobelimab was featured in multiple sessions. Collectively, these data highlighted the utility of vilobelimab in treating multiple inflammatory conditions, including PG and HS, with supporting evidence from clinical efficacy data, safety assessments, and pharmacokinetic and pharmacodynamic analyses. GOHIBIC (vilobelimab) granted EU marketing authorization In January 2025, the European Commission (EC) granted marketing authorization under exceptional circumstances for GOHIBIC (vilobelimab) for the treatment of adult patients with SARS-CoV-2-induced ARDS who are receiving systemic corticosteroids as part of standard of care and receiving invasive mechanical ventilation (IMV) with or without extracorporeal membrane oxygenation (ECMO). GOHIBIC (vilobelimab) is the first and only treatment approved in the European Union (EU) for the treatment of SARS-CoV-2-induced ARDS. The marketing authorization under exceptional circumstances for GOHIBIC (vilobelimab) is valid in all 27 EU member states as well as Iceland, Liechtenstein, and Norway. InflaRx is considering commercial partnering and distribution options in the EU and does not expect this approach will have a materially negative impact on its cash burn rate. In June 2024, InflaRx announced that GOHIBIC (vilobelimab) had been selected by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response within the U.S. Department of Health and Human Services, as one of three investigational therapies to be assessed in a Phase 2 clinical platform study exploring potential new options for the treatment of ARDS. Vilobelimab is one of three host-directed investigational drugs assessed in this study, with the safety and efficacy of each investigational drug to be studied in its own patient cohort and compared against placebo. This Phase 2 platform study is expected to collect data in order to define subsets of patients with ARDS who may benefit from specific host-directed therapeutics and to inform the design of potential Phase 3 studies. Dr. Thomas Taapken, Chief Financial Officer of InflaRx, said: “With our recent successful capital raise and the efficient utilization of our balance sheet, InflaRx is on solid financial footing, enabling us to efficiently advance our key development programs. Importantly, our cash runway into 2027 should allow us to reach several value inflection points, including the end-of-May interim analysis for the vilobelimab Phase 3 study in pyoderma gangrenosum, along with the eventual readout of this trial, as well as the INF904 Phase 2a topline data this summer.” Financing activities In February 2025, the company completed an underwritten public offering of ordinary shares and pre-funded warrants leading to gross proceeds from the offering of €28.7 million ($30.0 million), before deducting the underwriting discount and offering expenses. 2024 Financial highlightsRevenue In 2024, we realized revenues from product sales of GOHIBIC (vilobelimab) in the amount of €0.2 million, which represents an increase of €0.1 million compared to the prior year. Revenues reported are sales to end customers (hospitals). Sales to distributors do not constitute revenue for the Company. All revenues are attributed to sales made in the United States. Cost of sales Cost of sales increased by €2.8 million in 2024 compared to the corresponding costs for 2023, primarily due to higher inventory write-downs of €2.8 million as a result of quantities on hand exceeding quantities expected to be sold prior to expiry. Marketing and sales expenses Marketing and sales expenses increased by €2.8 million in 2024 compared to 2023, as 2024 represented the first full year of commercial efforts for GOHIBIC (vilobelimab) in the United States. In 2023, marketing and sales expenses were only incurred during the second half of the year. Research and development expenses Research and development expenses decreased by €5.7 million to €35.4 million in 2024 compared to the year 2023. This decrease was primarily attributable to €8.7 million lower third-party costs from manufacturing development activities and from clinical trials, offset by €1.6 million higher personnel expenses and €1.4 million higher other expenses compared to the previous year. Other expenses include a one-off milestone contractual payment of $1.0 million (€1.0 million) relating to market authorization for GOHIBIC (vilobelimab) in the EU. General and administrative expenses General and administrative expenses increased by €0.4 million to €13.0 million for the year ended December 31, 2024, from €12.6 million for the year ended December 31, 2023. This increase is comprised of higher personnel expenses of €0.9 million and partially offset by a decrease in legal and consulting expenses of €0.4 million and a decrease in insurance expenses of €0.4 million. Other income Other income decreased by €7.9 million in 2024 compared to the year 2023 due primarily to lower income from government grants. In June 2023, our grant from the German Ministry of Education and Research and the German Ministry of Health to support the development of vilobelimab for the treatment of severe COVID-19 patients ended. In 2024, upon qualifying for an allowance under the German Research Allowance Act, we recognized €5.1 million in income relating to expenses, eligible for reimbursement, which were incurred in the years 2020 to 2024. We remain eligible for additional research allowances for eligible expenses to be incurred from 2025 to 2027. Net financial result Net financial result increased by €4.7 million to a gain of €6.9 million in 2024 compared to €2.2 million in 2023. This overall net increase is mainly attributable to an increase of €5.5 million in foreign exchange results, partially offset by €0.6 million lower interest income from marketable securities compared to 2023. Net loss We incurred a net loss of €46.1 million, or €0.78 per ordinary share, in 2024, compared to €42.7 million, or €0.78 per ordinary share, in 2023. Liquidity and capital resources As of December 31, 2024, our total funds available amounted to approximately €55.2 million, comprised of €18.4 million of cash and cash equivalents and €36.8 million of marketable securities. Net cash used in operating activities Net cash used in operating activities increased to €48.6 million in 2024, from €37.8 million in 2023, mainly due to lower income recognized from German federal government grants and research allowances Additional financial information Additional information regarding these results and other relevant information is included in the notes to the financial statements in “Item 18. Financial Statements”, which are included in InflaRx’s most recent annual report on Form 20-F as filed today with the U.S. Securities and Exchange Commission (SEC). InflaRx N.V. and subsidiariesConsolidated statements of operations and comprehensive lossfor the years ended December 31, 2024, 2023 and 2022 2024 2023 2022 (in €, except for share data)
Revenues 165,789 63,089 — Cost of sales (3,317,039) (532,262) — Gross profit (3,151,250) (469,173) — Marketing and sales expenses (6,756,595) (4,001,299) — Research and development expenses (35,363,897) (41,024,131) (37,526,090)General and administrative expenses (13,024,441) (12,628,756) (14,869,564)Other income 5,287,616 13,219,704 20,159,169 Other expenses (297) (4,440) (1,381)Operating result (53,008,864) (44,908,096) (32,237,866)Finance income 3,196,813 3,804,827 608,679 Finance expenses (20,655) (35,628) (45,250)Foreign exchange result 3,670,235 (1,841,872) 2,442,297 Other financial result 103,285 313,240 (252,471)Income taxes (5,217) — — Loss for the period (46,064,402) (42,667,529) (29,484,611)Other comprehensive income (loss) that may be reclassified to profit or loss in subsequent periods:
Exchange differences on translation of foreign currency 58,344 125,085 4,206,810 TOTAL COMPREHENSIVE LOSS (46,006,058) (42,542,444) (25,277,801)
Share information
Weighted average number of shares outstanding 58,918,678 54,940,137 44,207,873 Loss per share (basic/diluted) (0.78) (0.78) (0.67)
InflaRx N.V. and subsidiariesConsolidated statements of financial position as of December 31, 2024 and 2023 December 31, 2024 December 31, 2023ASSETS (in €)Non-current assets Property and equipment 256,280 289,577 Right-of-use assets 758,368 1,071,666 Intangible assets 50,781 68,818 Other assets 204,233 257,267 Financial assets 3,092,290 9,052,741 Total non-current assets 4,361,952 10,740,069 Current assets Inventories 6,897,666 11,367,807 Current other assets 5,103,402 4,036,649 Other assets from government grants and research allowance 5,081,772 — Tax receivable 1,735,335 3,791,564 Other financial assets 34,462,352 77,504,518 Cash and cash equivalents 18,375,979 12,767,943 Total current assets 71,656,505 109,468,482 TOTAL ASSETS 76,018,457 120,208,551
EQUITY AND LIABILITIES Equity Issued capital 7,122,205 7,065,993 Share premium 334,929,685 334,211,338 Other capital reserves 44,115,861 40,050,053 Accumulated deficit (332,192,221) (286,127,819)Other components of equity 7,440,510 7,382,166 Total equity 61,416,039 102,581,730 Non-current liabilities Lease liabilities 399,066 745,716 Other liabilities 36,877 36,877 Total non-current liabilities 435,943 782,593 Current liabilities Trade and other payables 11,394,232 11,974,362 Lease liabilities 406,020 374,329 Employee benefits 2,064,678 1,609,766 Other liabilities 301,544 2,885,772 Total current liabilities 14,166,475 16,844,228 Total liabilities 14,602,417 17,626,822 TOTAL EQUITY AND LIABILITIES 76,018,457 120,208,552 InflaRx N.V. and subsidiariesConsolidated statements of changes in shareholders’ equityfor the years ended December 31, 2024, 2023 and 2022 in €Issuedcapital Sharepremium Othercapitalreserves Accumulateddeficit Othercomponentsof equity Totalequity
Balance as of January 01, 20225,304,452 280,310,744 30,591,209 (213,975,679) 3,050,270 105,280,996 Loss for the Period— — — (29,484,611) — (29,484,611)Exchange differences on translation of foreign currency— — — — 4,206,810 4,206,810 Total Comprehensive Loss— — — (29,484,611) 4,206,810 (25,277,801)Issuance of ordinary shares60,000 2,289,624 — — — 2,349,624 Transaction costs— (47,735) — — — (47,735)Equity-settled share-based payments— — 6,044,356 — — 6,044,356 Balance as of December 31, 20225,364,452 282,552,633 36,635,564 (243,460,290) 7,257,080 88,349,440 Loss for the Period— — — (42,667,529) — (42,667,529)Exchange differences on translation of foreign currency— — — — 125,085 125,085 Total Comprehensive Loss— — — (42,667,529) 125,085 (42,542,444)Issuance of ordinary shares1,687,110 54,796,819 — — — 56,483,929 Transaction costs— (3,360,626) — — — (3,360,626)Equity-settled share-based payments— — 3,414,489 — — 3,414,489 Share options exercised14,431 222,512 — — — 236,943 Balance as of December 31, 20237,065,993 334,211,338 40,050,053 (286,127,819) 7,382,166 102,581,730 Loss for the Period— — — (46,064,402) — (46,064,402)Exchange differences on translation of foreign currency— — — — 58,344 58,344 Total Comprehensive Loss— — — (46,064,402) 58,344 (46,006,058)Issuance of ordinary shares56,213 1,042,076 — — — 1,098,289 Transaction costs— (323,729) — — — (323,729)Equity-settled share-based payments— — 4,065,807 — — 4,065,807 Balance as of December 31, 20247,062,206 334,929,685 44,115,861 (332,192,221) 7,440,510 61,416,039
InflaRx N.V. and subsidiariesConsolidated statements of cash flowsfor the years ended December 31, 2024, 2023 and 2022 2024 2023 2022 (in €)Operating activities
Loss for the period (46,064,402) (42,667,529) (29,484,611)Adjustments for:
Depreciation & amortization of property and equipment, right-of-use assets and intangible assets 485,114 567,780 596,597 Net finance income (6,949,679) (2,240,566) (2,753,255)Share-based payment expense 4,065,807 3,414,489 6,044,356 Net foreign exchange differences (37,101) 413,017 385,359
Changes in:
Other assets from government grants and research allowances (5,081,772) 732,971 (732,971)Other assets 1,042,513 7,825,181 (3,308,485)Employee benefits 454,912 297,518 (64,024)Other liabilities (2,584,228) 2,738,164 9,403 Liabilities from government grants received — (6,209,266) (2,090,734)Trade and other payables (580,129) 6,986,824 (3,586,706)Inventories 4,470,141 (11,367,807) — Interest received 2,243,197 1,732,284 1,287,200 Interest paid (21,064) (36,025) (44,946)Net cash used in operating activities (48,556,690) (37,812,966) (33,742,817)Investing activities
Purchase of intangible assets and property and equipment (46,871) (81,100) (162,391)Purchase of current and non-current financial assets (35,340,107) (104,051,972) (64,474,543)Proceeds from the maturity of current financial assets 87,751,331 86,436,456 83,995,029 Net cash from/ (used in) investing activities 52,364,354 (17,696,616) 19,358,095 Financing activities
Proceeds from issuance of ordinary shares 1,098,289 56,483,929 2,349,624 Transaction costs from issuance of ordinary shares (323,729) (3,360,626) (47,735)Proceeds from exercise of share options — 236,943 — Repayment of lease liabilities (388,114) (373,977) (364,430)Net cash from financing activities 386,446 52,986,269 1,937,459 Net in-/decrease in cash and cash equivalents 4,194,110 (2,523,313) (12,447,262)Effect of exchange rate changes on cash and cash equivalents 1,413,926 (974,099) 2,462,622 Cash and cash equivalents at beginning of period 12,767,943 16,265,355 26,249,995 Cash and cash equivalents at end of period 18,375,979 12,767,943 16,265,355 About GOHIBIC (vilobelimab) In the EU, GOHIBIC (vilobelimab) has been granted marketing authorization under exceptional circumstances for the treatment of adult patients with SARS-CoV-2-induced acute respiratory distress syndrome (ARDS) who are receiving systemic corticosteroids as part of standard of care and receiving invasive mechanical ventilation (IMV) (with or without extracorporeal membrane oxygenation (ECMO)). The EU approval of GOHIBIC (vilobelimab) is supported by the previously announced results of the multicenter Phase 3 PANAMO trial, one of the largest 1:1 randomized, double-blind, placebo-controlled trials in invasively mechanically ventilated COVID-19 patients in intensive care units. The results showed that vilobelimab treatment improved survival with a relative reduction in 28-day all-cause mortality of 23.9% compared to placebo in the global data set. The data were published in The Lancet Respiratory Medicine. A marketing authorization under exceptional circumstances is recommended when the benefit/risk assessment is determined to be positive but, due to the rarity of the disease, it’s unlikely that comprehensive data can be obtained under normal conditions of use. Under the terms of GOHIBIC (vilobelimab)’s approval in the EC, InflaRx will provide annual updates to EMA on the previously announced clinical platform study planned by the Biomedical Advanced Research and Development Authority (BARDA). Vilobelimab is included in this study as one of three new potential therapies for treating ARDS. In the U.S., GOHIBIC (vilobelimab) has been granted an Emergency Use Authorization by the Food and Drug Administration (FDA) for the treatment of COVID-19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO. The emergency use of GOHIBIC (vilobelimab) is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization revoked sooner. GOHIBIC (vilobelimab) is an investigational drug that has not been approved by the FDA for any indication, including for the treatment of COVID-19. There is limited information known about the safety and effectiveness of using GOHIBIC (vilobelimab) to treat people in the hospital with COVID-19. Please see additional information in the Fact Sheet for Healthcare Providers, Fact Sheet for Patients and Parents/Caregivers and FDA Letter of Authorization on the GOHIBIC (vilobelimab) website http://www.gohibic.com. Important Safety Information about GOHIBIC (vilobelimab) There are limited clinical data available for GOHIBIC (vilobelimab). Serious and unexpected adverse events (AEs) may occur that have not been previously reported with GOHIBIC (vilobelimab) use. GOHIBIC (vilobelimab) has been associated with an increase of serious infections. In patients with COVID-19, monitor for signs and symptoms of new infections during and after treatment with GOHIBIC (vilobelimab). Hypersensitivity reactions have been observed with GOHIBIC (vilobelimab). If a severe hypersensitivity reaction occurs, administration of GOHIBIC (vilobelimab) should be discontinued and appropriate therapy initiated. The most common adverse reactions (incidence ≥3%) are pneumonia, sepsis, delirium, pulmonary embolism, hypertension, pneumothorax, deep vein thrombosis, herpes simplex, enterococcal infection, bronchopulmonary aspergillosis, hepatic enzyme increased, urinary tract infection, hypoxia, thrombocytopenia, pneumomediastinum, respiratory tract infection, supraventricular tachycardia, constipation, and rash. Healthcare providers and/or their designee are responsible for mandatory FDA MedWatch reporting of all medication errors and serious AEs or deaths that occur during GOHIBIC (vilobelimab) treatment and are considered to be potentially attributable to GOHIBIC (vilobelimab). Report side effects to the FDA at 1-800-FDA-1088 or www.FDA.gov/medwatch. In addition, side effects can be reported to InflaRx at: pvusa@inflarx.de. For the full prescribing information and additional important safety information, please visit www.GOHIBIC.com. The COVID-19 related work described herein was partly funded by the German Federal Government through grant number 16LW0113 (VILO-COVID). All responsibility for the content of this work lies with InflaRx. About vilobelimab Vilobelimab is a first-in-class monoclonal anti-human complement factor C5a antibody, which highly and effectively blocks the biological activity of C5a and demonstrates high selectivity towards its target in human blood. Thus, vilobelimab leaves the formation of the membrane attack complex (C5b-9) intact as an important defense mechanism of the innate immune system, which is not the case for molecules blocking C5. In pre-clinical studies, vilobelimab has been shown to control the inflammatory response-driven tissue and organ damage by specifically blocking C5a as a key “amplifier” of this response. Vilobelimab is being developed for various debilitating or life-threatening inflammatory indications, including pyoderma gangrenosum (PG). Vilobelimab has been granted orphan drug designation for the treatment of PG by both the FDA and the EMA, as well as fast track designation by the FDA. About INF904 INF904 is an orally administered, small molecule inhibitor of the C5a receptor that has shown anti-inflammatory therapeutic effects in several pre-clinical disease models. Further, in contrast to the marketed C5aR inhibitor, in vitro experiments demonstrated that INF904 has minimal inhibition of the cytochrome P450 3A4/5 (CYP3A4/5) enzymes, which play an important role in the metabolism of a variety of metabolites and drugs, including glucocorticoids. Reported results from a first-in-human study demonstrated that INF904 is well tolerated in treated subjects and exhibits no safety signals of concern in single doses ranging from 3 mg to 240 mg or multiple doses ranging from 30 mg once per day (QD) to 90 mg twice per day (BID) for 14 days. PK / pharmacodynamic data support the best-in-class potential of INF904 with a ≥90% blockade of C5a-induced neutrophil activation achieved over the 14-day dosing period. About InflaRx N.V. InflaRx GmbH (Germany) and InflaRx Pharmaceuticals Inc. (USA) are wholly owned subsidiaries of InflaRx N.V. (together, InflaRx). InflaRx (Nasdaq: IFRX) is a biopharmaceutical company pioneering anti-inflammatory therapeutics by applying its proprietary anti-C5a and anti-C5aR technologies to discover, develop and commercialize highly potent and specific inhibitors of the complement activation factor C5a and its receptor C5aR. C5a is a powerful inflammatory mediator involved in the progression of a wide variety of inflammatory diseases. InflaRx’s lead product candidate, vilobelimab, is a novel, intravenously delivered, first-in-class, anti-C5a monoclonal antibody that selectively binds to free C5a and has demonstrated disease-modifying clinical activity and tolerability in multiple clinical studies in different indications. InflaRx is also developing INF904, an orally administered small molecule inhibitor of C5a-induced signaling via the C5a receptor. InflaRx was founded in 2007, and the group has offices and subsidiaries in Jena and Munich, Germany, as well as Ann Arbor, MI, USA. For further information, please visit www.inflarx.de. Contacts: InflaRx N.V.MC Services AGJan Medina, CFAVice President, Head of Investor RelationsEmail: IR@inflarx.deKatja Arnold, Laurie Doyle, Dr. Regina LutzEmail: inflarx@mc-services.eu Europe: +49 89-210 2280U.S.: +1-339-832-0752 * Eligibility Requirements, Terms and Conditions apply. Please see the full Terms and Conditions provided on the webpage: The InflaRx Commitment Program. FORWARD-LOOKING STATEMENTS This press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue,” among others. Forward-looking statements appear in a number of places throughout this release and may include statements regarding our intentions, beliefs, projections, outlook, analyses and current expectations concerning, among other things, the receptiveness of GOHIBIC (vilobelimab) as a treatment for COVID-19 by COVID-19 patients and U.S. hospitals and related treatment recommendations by medical/healthcare institutes and other third-party organizations, our ability to successfully commercialize and the receptiveness of GOHIBIC (vilobelimab) as a treatment for COVID-19 by COVID-19 patients and U.S. hospitals or our other product candidates; our expectations regarding the size of the patient populations for, market opportunity for, coverage and reimbursement for, estimated returns and return accruals for, and clinical utility of GOHIBIC (vilobelimab) in its approved or authorized indications or for vilobelimab and any other product candidates, under an EUA and in the future if approved for commercial use in the U.S. or elsewhere; our ability to successfully implement The InflaRx Commitment Program, the success of our future clinical trials for vilobelimab’s treatment of COVID-19 and other debilitating or life-threatening inflammatory indications, including PG, and any other product candidates, including INF904, and whether such clinical results will reflect results seen in previously conducted pre-clinical studies and clinical trials; the timing, progress and results of pre-clinical studies and clinical trials of our product candidates and statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, the costs of such trials and our research and development programs generally; our interactions with regulators regarding the results of clinical trials and potential regulatory approval pathways, including related to our biologics license application submission for GOHIBIC (vilobelimab), and our ability to obtain and maintain full regulatory approval of vilobelimab or GOHIBIC (vilobelimab) for any indication; whether the FDA, or any comparable foreign regulatory authority will accept or agree with the number, design, size, conduct or implementation of our clinical trials, including any proposed primary or secondary endpoints for such trials; our expectations regarding the scope of any approved indication for vilobelimab; our ability to leverage our proprietary anti-C5a and C5aR technologies to discover and develop therapies to treat complement-mediated autoimmune and inflammatory diseases; our ability to protect, maintain and enforce our intellectual property protection for vilobelimab and any other product candidates, and the scope of such protection; our manufacturing capabilities and strategy, including the scalability and cost of our manufacturing methods and processes and the optimization of our manufacturing methods and processes, and our ability to continue to rely on our existing third-party manufacturers and our ability to engage additional third-party manufacturers for our planned future clinical trials and for commercial supply of vilobelimab and for the finished product GOHIBIC (vilobelimab); our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for or ability to obtain additional financing; our ability to defend against liability claims resulting from the testing of our product candidates in the clinic or, if approved, any commercial sales; if any of our product candidates obtain regulatory approval, our ability to comply with and satisfy ongoing obligations and continued regulatory overview; our ability to comply with enacted and future legislation in seeking marketing approval and commercialization; our future growth and ability to compete, which depends on our retaining key personnel and recruiting additional qualified personnel; and our competitive position and the development of and projections relating to our competitors in the development of C5a and C5aR inhibitors or our industry; and the risks, uncertainties and other factors described under the heading “Risk Factors” in our periodic filings with the SEC. These statements speak only as of the date of this press release and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and we assume no obligation to update these forward-looking statements, even if new information becomes available in the future, except as required by law.
临床2期孤儿药临床3期上市批准快速通道
2025-01-15
Jena, Germany, January 15, 2025– InflaRx N.V. (Nasdaq: IFRX), a biopharmaceutical company pioneering anti-inflammatory therapeutics targeting the complement system, today announced that the European Commission (EC) has granted marketing authorization under exceptional circumstances for GOHIBIC® (vilobelimab) for the treatment of adult patients with SARS-CoV-2-induced acute respiratory distress syndrome (ARDS) who are receiving systemic corticosteroids as part of standard of care and receiving invasive mechanical ventilation (IMV) with or without extracorporeal membrane oxygenation (ECMO). GOHIBIC is the first and only treatment approved in the European Union for the treatment of SARS-CoV-2-induced ARDS.
Prof. Niels C. Riedemann, Chief Executive Officer and Founder of InflaRx, commented: “The European Commission’s approval of GOHIBIC, the first approval of its kind, reflects our commitment to ICU patients with SARS-CoV-2-induced ARDS, a pressing medical setting in need of more effective therapeutic options. I would like to thank the entire InflaRx team for its dedication and diligence resulting in this successful marketing authorization, and we are grateful for the support provided by the intensive care physicians, patients and their families who participated in the PANAMO study which supported the marketing authorization application.”
The marketing authorization under exceptional circumstances for GOHIBIC is valid in all 27 EU member states as well as Iceland, Liechtenstein, and Norway. InflaRx is considering commercial partnering and distribution options in the EU and does not expect this approach will have a meaningfully negative impact on its cash burn rate.
Important Information about GOHIBIC (vilobelimab)
In the EU, GOHIBIC (vilobelimab) has been granted marketing authorization under exceptional circumstances for the treatment of adult patients with SARS-CoV-2-induced acute respiratory distress syndrome (ARDS) who are receiving systemic corticosteroids as part of standard of care and receiving invasive mechanical ventilation (IMV) (with or without extracorporeal membrane oxygenation (ECMO)). The EU approval of GOHIBIC is supported by the previously announced results of the multicenter Phase 3 PANAMO trial, one of the largest 1:1 randomized, double-blind, placebo-controlled trials in invasively mechanically ventilated COVID-19 patients in intensive care units. The results showed that vilobelimab treatment improved survival with a relative reduction in 28-day all-cause mortality of 23.9% compared to placebo in the global data set. The data were published in The Lancet Respiratory Medicine.
A marketing authorization under exceptional circumstances is recommended when the benefit/risk assessment is determined to be positive but, due to the rarity of the disease, it’s unlikely that comprehensive data can be obtained under normal conditions of use. Under the terms of GOHIBIC’s approval in the EC, InflaRx will provide annual updates to EMA on the previously announced clinical platform study planned by the Biomedical Advanced Research and Development Authority (BARDA). Vilobelimab is included in this study as one of three new potential therapies for treating ARDS.
In the U.S., GOHIBIC (vilobelimab) has been granted an Emergency Use Authorization by the Food and Drug Administration (FDA) for the treatment of COVID-19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO. The emergency use of GOHIBIC is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization revoked sooner.
GOHIBIC (vilobelimab) is an investigational drug that has not been approved by the FDA for any indication, including for the treatment of COVID-19. There is limited information known about the safety and effectiveness of using GOHIBIC to treat people in the hospital with COVID-19. Please see additional information in the Fact Sheet for Healthcare Providers, Fact Sheet for Patients and Parents/Caregivers and FDA Letter of Authorization on the GOHIBIC website http://www.gohibic.com.
Important Safety Information about GOHIBIC (vilobelimab)
There are limited clinical data available for GOHIBIC. Serious and unexpected adverse events (AEs) may occur that have not been previously reported with GOHIBIC use.
GOHIBIC has been associated with an increase of serious infections. In patients with COVID-19, monitor for signs and symptoms of new infections during and after treatment with GOHIBIC. Hypersensitivity reactions have been observed with GOHIBIC. If a severe hypersensitivity reaction occurs, administration of GOHIBIC should be discontinued and appropriate therapy initiated.
The most common adverse reactions (incidence ≥3%) are pneumonia, sepsis, delirium, pulmonary embolism, hypertension, pneumothorax, deep vein thrombosis, herpes simplex, enterococcal infection, bronchopulmonary aspergillosis, hepatic enzyme increased, urinary tract infection, hypoxia, thrombocytopenia, pneumomediastinum, respiratory tract infection, supraventricular tachycardia, constipation, and rash.
Healthcare providers and/or their designee are responsible for mandatory FDA MedWatch reporting of all medication errors and serious AEs or deaths that occur during GOHIBIC treatment and are considered to be potentially attributable to GOHIBIC.
Report side effects to the FDA at 1-800-FDA-1088 or www.FDA.gov/medwatch. In addition, side effects can be reported to InflaRx at: pvusa@inflarx.de.
For the full prescribing information and additional important safety information, please visit www.GOHIBIC.com.
About Vilobelimab
Vilobelimab is a first-in-class monoclonal anti-human complement factor C5a antibody, which highly and effectively blocks the biological activity of C5a and demonstrates high selectivity towards its target in human blood. Thus, vilobelimab leaves the formation of the membrane attack complex (C5b-9) intact as an important defense mechanism of the innate immune system, which is not the case for molecules blocking C5. In pre-clinical studies, vilobelimab has been shown to control the inflammatory response-driven tissue and organ damage by specifically blocking C5a as a key “amplifier” of this response.
Vilobelimab is being developed for various debilitating or life-threatening inflammatory indications, including pyoderma gangrenosum (PG). Vilobelimab has been granted orphan drug designation for the treatment of PG by both the FDA and the EMA, as well as fast track designation by the FDA.
The COVID-19 related work described herein is partly funded by the German Federal Government through grant number 16LW0113 (VILO-COVID). All responsibility for the content of this work lies with InflaRx.
About InflaRx
InflaRx (Nasdaq: IFRX) is a biopharmaceutical company pioneering anti-inflammatory therapeutics by applying its proprietary anti-C5a and anti-C5aR technologies to discover, develop and commercialize highly potent and specific inhibitors of the complement activation factor C5a and its receptor C5aR. C5a is a powerful inflammatory mediator involved in the progression of a wide variety of inflammatory diseases. InflaRx’s lead product candidate, vilobelimab, is a novel, intravenously delivered, first-in-class, anti-C5a monoclonal antibody that selectively binds to free C5a and has demonstrated disease-modifying clinical activity and tolerability in multiple clinical studies in different indications. InflaRx is also developing INF904, an orally administered small molecule inhibitor of the C5a receptor. InflaRx was founded in 2007, and the group has offices and subsidiaries in Jena and Munich, Germany, as well as Ann Arbor, MI, USA. For further information, please visit www.inflarx.com.
InflaRx GmbH (Germany) and InflaRx Pharmaceuticals Inc. (USA) are wholly owned subsidiaries of InflaRx N.V. (together, InflaRx).
Contacts:
FORWARD-LOOKING STATEMENTS
This press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue,” among others. Forward-looking statements appear in a number of places throughout this release and may include statements regarding our intentions, beliefs, projections, outlook, analyses, current expectations and the risks, uncertainties and other factors described under the heading “Risk Factors” and “Cautionary statement regarding forward looking statements” in our periodic filings with the U.S. Securities and Exchange Commission. These statements speak only as of the date of this press release and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and we assume no obligation to update these forward-looking statements, even if new information becomes available in the future, except as required by law.
Download PDF
临床结果上市批准孤儿药临床3期快速通道
2024-11-15
JENA, Germany, Nov. 15, 2024 (GLOBE NEWSWIRE) -- InflaRx N.V. (Nasdaq: IFRX), a biopharmaceutical company pioneering anti-inflammatory therapeutics targeting the complement system, today announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion recommending marketing authorization of GOHIBIC (vilobelimab), under exceptional circumstances for the treatment of adult patients with SARS-CoV-2-induced acute respiratory distress syndrome (ARDS) who are receiving systemic corticosteroids as part of standard of care and receiving invasive mechanical ventilation (IMV) (with or without extracorporeal membrane oxygenation (ECMO)). The Company expects the European Commission to adopt the positive opinion and issue a marketing authorization within 67 days. Prof. Niels C. Riedemann, Chief Executive Officer and Founder of InflaRx, commented: “The positive CHMP opinion reflects a significant milestone in the development of our anti-C5a antibody vilobelimab, and, together with the previously received Emergency Use Authorization granted by the FDA, further validates its therapeutic potential. Patients in the ICU continue to die from SARS-CoV-2-induced ARDS, an important reminder of the ongoing need for more effective treatments for these patients. We are grateful to the intensive care physicians and patients’ families who worked with InflaRx on the PANAMO study.” A marketing authorization under exceptional circumstances is recommended when the benefit/risk assessment is determined to be positive but, due to the rarity of the disease, it’s unlikely that comprehensive data can be obtained under normal conditions of use. Under the terms of GOHIBIC’s approval in the EU, which is anticipated early next year, InflaRx will provide annual updates to EMA on the previously announced clinical platform study planned by the Biomedical Advanced Research and Development Authority (BARDA). Vilobelimab is included in this study as one of three new potential therapies for treating ARDS. InflaRx plans to commercialize the product in Europe under its proprietary brand name GOHIBIC®. As previously indicated, InflaRx is considering commercial distribution options with potential partners in the EU. InflaRx does not expect this approach will have a meaningfully negative impact on its cash burn rate. The positive CHMP opinion is supported by the previously announced results of the multicenter Phase 3 PANAMO trial, one of the largest 1:1 randomized, double-blind, placebo-controlled trials in invasively mechanically ventilated COVID-19 patients in intensive care units. The results showed that vilobelimab treatment improved survival with a relative reduction in 28-day all-cause mortality of 23.9% compared to placebo in the global data set. The data were published in The Lancet Respiratory Medicine. Important Information about GOHIBIC (vilobelimab)GOHIBIC (vilobelimab) has been granted an Emergency Use Authorization by the U.S. Food and Drug Administration (FDA) for the treatment of COVID-19 in hospitalized adults when initiated within 48 hours of receiving IMV or ECMO. The emergency use of GOHIBIC is only authorized for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of drugs and biological products during the COVID-19 pandemic under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization revoked sooner. GOHIBIC (vilobelimab) is an investigational drug that has not been approved by the FDA for any indication, including for the treatment of COVID-19. There is limited information known about the safety and effectiveness of using GOHIBIC to treat people in the hospital with COVID-19. Please see additional information in the Fact Sheet for Healthcare Providers, Fact Sheet for Patients and Parents/Caregivers and FDA Letter of Authorization on the GOHIBIC website (www.GOHIBIC.com). Important Safety Information about GOHIBIC (vilobelimab)There are limited clinical data available for GOHIBIC. Serious and unexpected adverse events (AEs) may occur that have not been previously reported with GOHIBIC use. GOHIBIC has been associated with an increase of serious infections. In patients with COVID-19, monitor for signs and symptoms of new infections during and after treatment with GOHIBIC. Hypersensitivity reactions have been observed with GOHIBIC. If a severe hypersensitivity reaction occurs, administration of GOHIBIC should be discontinued and appropriate therapy initiated. The most common adverse reactions (incidence ≥3%) are pneumonia, sepsis, delirium, pulmonary embolism, hypertension, pneumothorax, deep vein thrombosis, herpes simplex, enterococcal infection, bronchopulmonary aspergillosis, hepatic enzyme increased, urinary tract infection, hypoxia, thrombocytopenia, pneumomediastinum, respiratory tract infection, supraventricular tachycardia, constipation, and rash. Healthcare providers and/or their designee are responsible for mandatory FDA MedWatch reporting of all medication errors and serious AEs or deaths that occur during GOHIBIC treatment and are considered to be potentially attributable to GOHIBIC. Report side effects to the FDA at 1-800-FDA-1088 or www.FDA.gov/medwatch. In addition, side effects can be reported to InflaRx at: pvusa@inflarx.de. For the full prescribing information and additional important safety information, please visit www.GOHIBIC.com. About VilobelimabVilobelimab is a first-in-class monoclonal anti-human complement factor C5a antibody, which highly and effectively blocks the biological activity of C5a and demonstrates high selectivity towards its target in human blood. Thus, vilobelimab leaves the formation of the membrane attack complex (C5b-9) intact as an important defense mechanism of the innate immune system, which is not the case for molecules blocking C5. In pre-clinical studies, vilobelimab has been shown to control the inflammatory response-driven tissue and organ damage by specifically blocking C5a as a key “amplifier” of this response. In addition to development in COVID-19, vilobelimab is also being developed for various debilitating or life-threatening inflammatory indications, including pyoderma gangrenosum (PG). Vilobelimab has been granted orphan drug designation for the treatment of PG by both the FDA and the EMA, as well as fast track designation by the FDA. About InflaRxInflaRx (Nasdaq: IFRX) is a biopharmaceutical company pioneering anti-inflammatory therapeutics by applying its proprietary anti-C5a and anti-C5aR technologies to discover, develop and commercialize highly potent and specific inhibitors of the complement activation factor C5a and its receptor C5aR. C5a is a powerful inflammatory mediator involved in the progression of a wide variety of inflammatory diseases. InflaRx’s lead product candidate, vilobelimab, is a novel, intravenously delivered, first-in-class, anti-C5a monoclonal antibody that selectively binds to free C5a and has demonstrated disease-modifying clinical activity and tolerability in multiple clinical studies in different indications. InflaRx is also developing INF904, an orally administered small molecule inhibitor of the C5a receptor. InflaRx was founded in 2007, and the group has offices and subsidiaries in Jena and Munich, Germany, as well as Ann Arbor, MI, USA. For further information, please visit www.inflarx.com. InflaRx GmbH (Germany) and InflaRx Pharmaceuticals Inc. (USA) are wholly owned subsidiaries of InflaRx N.V. (together, InflaRx). Contacts: InflaRx N.V.MC Services AGJan Medina, CFAVice President, Head of Investor RelationsEmail: IR@inflarx.deKatja Arnold, Laurie Doyle, Dr. Regina LutzEmail: inflarx@mc-services.eu Europe: +49 89-210 2280U.S.: +1-339-832-0752 FORWARD-LOOKING STATEMENTSThis press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue,” among others. Forward-looking statements appear in a number of places throughout this release and may include statements regarding our intentions, beliefs, projections, outlook, analyses, current expectations and the risks, uncertainties and other factors described under the heading “Risk Factors” and “Cautionary statement regarding forward looking statements” in our periodic filings with the U.S. Securities and Exchange Commission. These statements speak only as of the date of this press release and involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements, and we assume no obligation to update these forward-looking statements, even if new information becomes available in the future, except as required by law.
临床结果孤儿药上市批准临床3期快速通道
分析
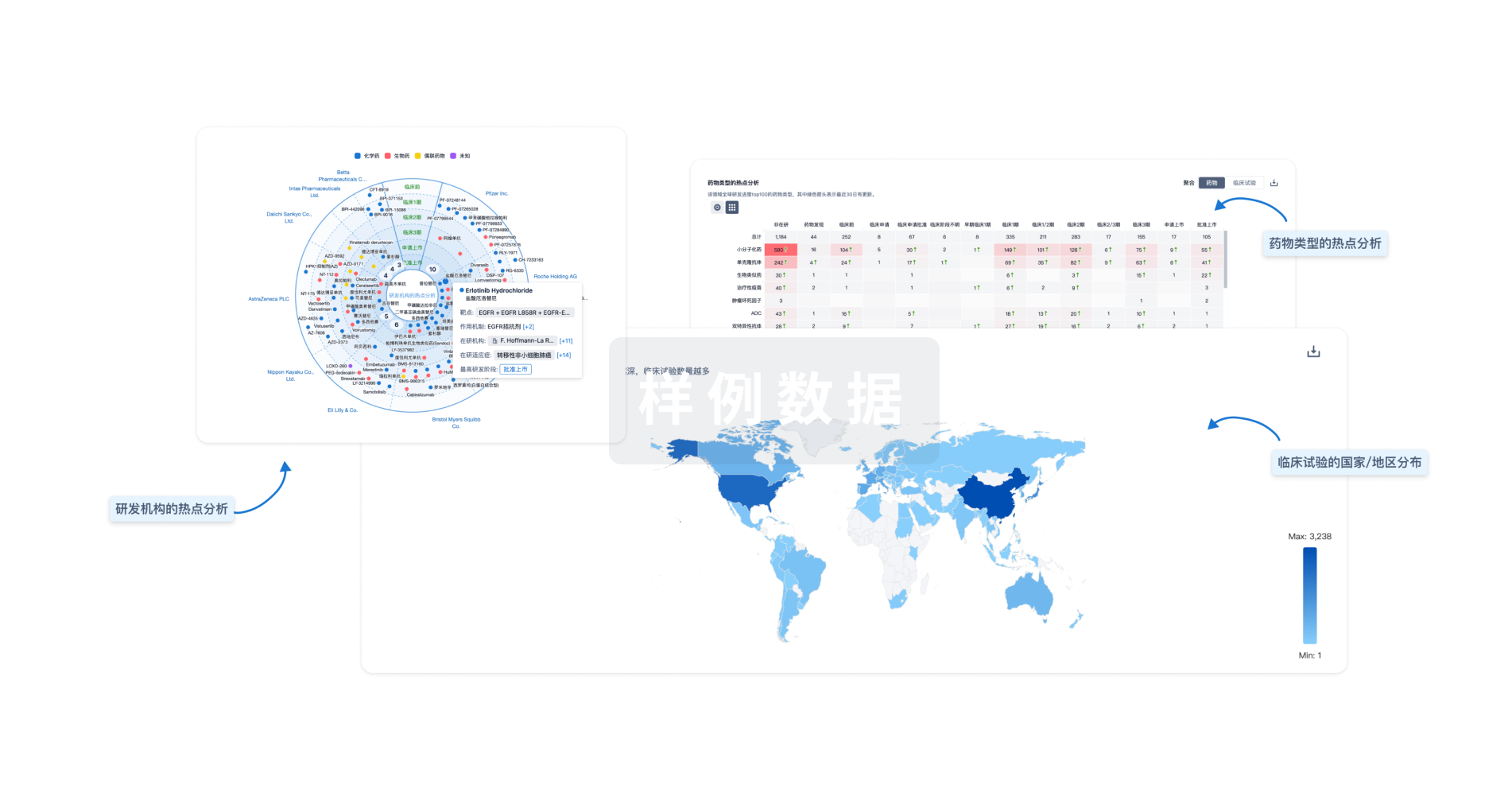
对领域进行一次全面的分析。
登录
或
Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


