评估阿伐曲泊帕治疗中国成人慢性免疫性血小板减少症(ITP)疗效和安全性: 多中心、随机、双盲、安慰剂对照,加扩展研究的 III 期临床试验
主要:确定第6周的血小板反应率,证明阿伐曲泊帕对中国成人慢性ITP患者的疗效优于安慰剂。次要:根据所测量的第8天的血小板反应,证明阿伐曲泊帕优效于安慰剂。根据6周内所测定血小板反应率累积周数(定义为:在无补救治疗情况下,血小板计数≥50×109/L的累计周数),证明阿伐曲泊帕优效于安慰剂。评估阿伐曲泊帕在减少出血和补救治疗方面的疗效优于安慰剂。评估阿伐曲泊帕长期治疗的有效性。评估阿伐曲泊帕安全性和耐受性。
100 项与 Carton Service, Inc. 相关的临床结果
0 项与 Carton Service, Inc. 相关的专利(医药)
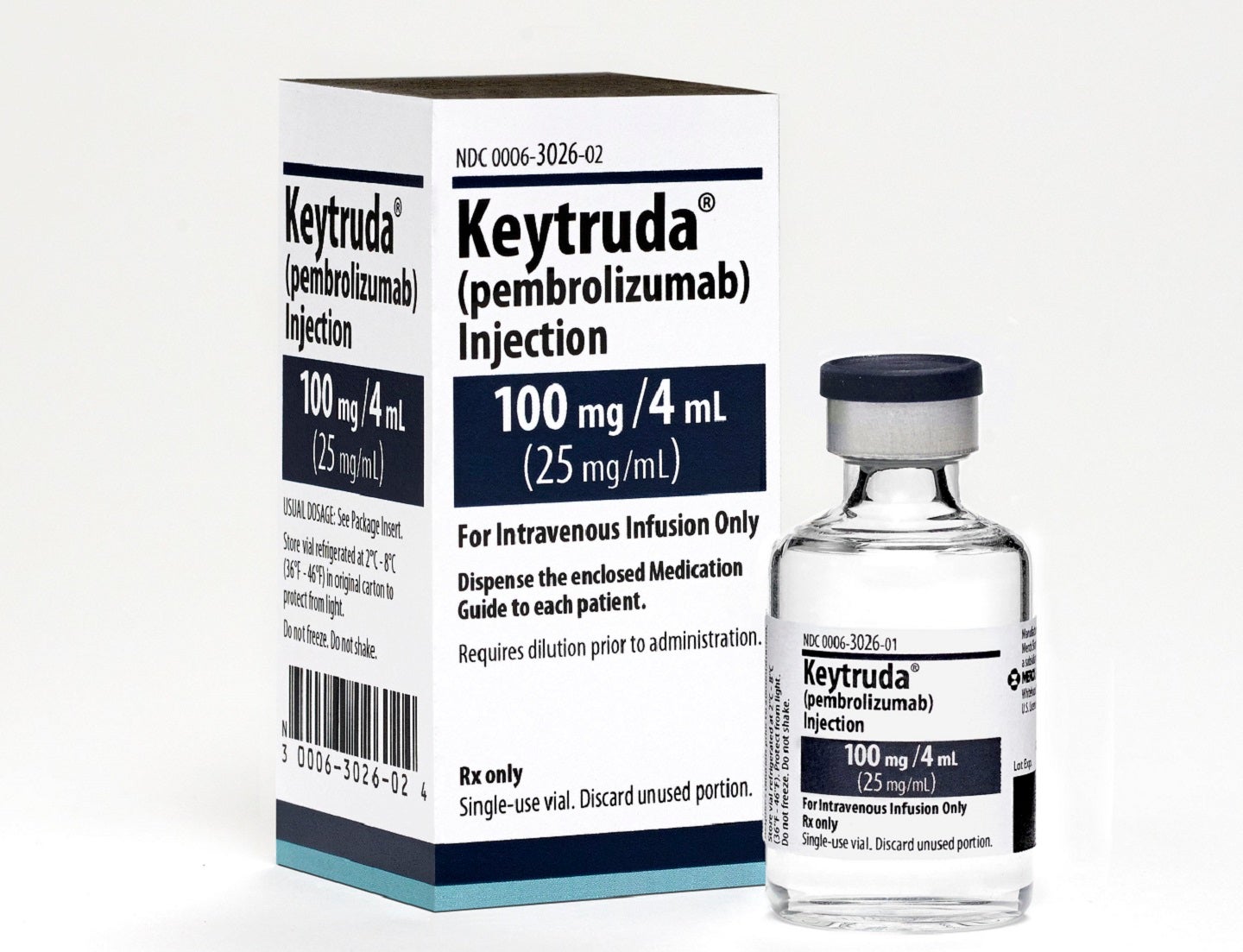
KEYTRUDA is an anti-PD-1 therapy developed by Merck. Credit: Copyright © 2023 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Keytruda 100mg/4mL Vial and Carton
2015
Merck
has secured approval from the US Food and Drug Administration (FDA) for
KEYTRUDA (pembrolizumab) in combination with Padcev (enfortumab vedotin-ejfv)
for first-line treatment of some adult patients with locally advanced or metastatic urothelial carcinoma (la/mUC).
KEYTRUDA is an anti-PD-1 therapy developed by
Merck
, while Padcev has been developed by
Astellas
and
Seagen
.
The combination therapy can be used to treat la/mUC patients who do not qualify for cisplatin-containing chemotherapy.
Merck stated that the regulatory approval represents the first of its kind received by an anti-PD-1 therapy in combination with an antibody-drug conjugate for use in targeted patients in the US.
It is supported by objective response rates (ORR) and median duration of response (DOR) in combined dose escalation/cohort A and cohort K of the
Phase lb/ll KEYNOTE-869 study, also known as the EV-103 trial
.
The trial was jointly conducted by
Seagen
and Astellas.
Results showed that patients treated with Padcev along with KEYTRUDA achieved a confirmed ORR of 68%. 12% experienced a complete response and 55% a partial response.
Merck Research Laboratories senior vice-president, global clinical development head and chief medical officer Dr Eliav Barr said: “This approval is a major milestone in the treatment of patients with locally advanced or metastatic urothelial carcinoma because it is the first approved combination of an immunotherapy and an antibody-drug conjugate for these patients.
“This expands the use of KEYTRUDA-based regimens to more patients with advanced urothelial carcinoma, and demonstrates the value of collaboration in creating new combination approaches for patients in need of more options.”
100 项与 Carton Service, Inc. 相关的药物交易
100 项与 Carton Service, Inc. 相关的转化医学








