更新于:2024-11-01

Shandong Keyuan Pharmaceutical Co., Ltd.
更新于:2024-11-01
概览
标签
耳鼻咽喉疾病
免疫系统疾病
呼吸系统疾病
小分子化药
疾病领域得分
一眼洞穿机构专注的疾病领域
暂无数据
技术平台
公司药物应用最多的技术
暂无数据
靶点
公司最常开发的靶点
暂无数据
| 排名前五的药物类型 | 数量 |
|---|---|
| 小分子化药 | 2 |
| 排名前五的靶点 | 数量 |
|---|---|
| H1 receptor(组胺H1受体) | 1 |
| IKKε x TBK1 | 1 |
关联
2
项与 山东科源制药股份有限公司 相关的药物作用机制 IKKε抑制剂 [+1] |
在研机构 |
在研适应症 |
非在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 美国 |
首次获批日期1996-12-17 |
作用机制 H1 receptor拮抗剂 |
非在研适应症 |
最高研发阶段批准上市 |
首次获批国家/地区 日本 |
首次获批日期1970-01-17 |
10
项与 山东科源制药股份有限公司 相关的临床试验恩格列净二甲双胍缓释片(25mg/1000mg)在中国健康受试者中空腹和餐后给药条件下随机、开放、单剂量、两制剂、两序列、两周期、双交叉生物等效性试验
按有关生物等效性试验的规定,选择Boehringer Ingelheim Pharmaceuticals, Inc持证,Patheon Pharmaceuticals Inc.生产的恩格列净二甲双胍缓释片(商品名:Synjardy® XR,规格:25mg/1000mg)为参比制剂,对山东力诺制药有限公司提供的受试制剂恩格列净二甲双胍缓释片(规格:25mg/1000mg)进行空腹和餐后给药人体生物等效性试验,比较受试制剂中药物的吸收速度和吸收程度与参比制剂的差异是否在可接受的范围内,评价两种制剂在空腹和餐后给药条件下的生物等效性。同时观察健康受试者口服受试制剂和参比制剂后的安全性。
开始日期2024-04-29 |
申办/合作机构 |
盐酸鲁拉西酮片在中国成年健康受试者中的一项单中心、随机、开放、空腹和餐后单次给药、两制剂、两序列、四周期、完全重复交叉生物等效性研究
主要研究目的:
考察单次口服(空腹/餐后)受试制剂盐酸鲁拉西酮片【规格:40 mg(按C28H36N4O2S?HCl计)】与参比制剂【商品名:罗舒达®,规格:40 mg(按C28H36N4O2S?HCl计)】在中国健康人体的相对生物利用度,分析两种制剂的药代动力学参数,评价两制剂的生物等效性,为该药的申报及临床用药提供参考依据。
次要研究目的:
评价单次口服(空腹/餐后)受试制剂和参比制剂在中国成年健康受试者中的安全性。
开始日期2024-02-20 |
申办/合作机构 |
中国健康受试者空腹及餐后状态下单次口服吡拉西坦片的单中心、开放、随机、两制剂、两序列、两周期、双交叉人体生物等效性试验
研究健康受试者空腹及餐后单次口服受试制剂吡拉西坦片(山东力诺制药有限公司,规格:0.8g)与参比制剂吡拉西坦片(商品名:Nootropil®,规格:0.8g)在吸收程度和速度方面是否存在差异,评价受试制剂和参比制剂在空腹及餐后条件下给药时的生物等效性,观察受试制剂和参比制剂在健康受试者中的安全性。
开始日期2023-09-27 |
申办/合作机构 |
100 项与 山东科源制药股份有限公司 相关的临床结果
登录后查看更多信息
0 项与 山东科源制药股份有限公司 相关的专利(医药)
登录后查看更多信息
3
项与 山东科源制药股份有限公司 相关的文献(医药)2023-12-01·Current Medicinal Chemistry
YF343, A Novel Histone Deacetylase Inhibitor, Combined with CQ
to Inhibit- Autophagy, Contributes to Increased Apoptosis in Triple-
Negative Breast Cancer
Article
作者: Qiu, Huiran ; Zhang, Jing ; Luo, Tingting ; Lu, Wen-xia ; Zhang, Hua ; Wu, Yong-mei ; Yang, Fei-fei ; Han, Li-na ; Lin, Yan ; Liu, Na ; Ge, Di ; Duan, Wen-qi
2022-09-01·Analytica Chimica Acta
An extracellular matrix biosensing mimetic for evaluating cathepsin as a host target for COVID-19
Article
作者: Wang, Ying ; Hu, Jianguo ; Zhou, Lei ; Li, Hao ; Li, Jinlong ; Liu, Hongkai ; Hou, Wenmin ; Lin, Xia ; Liu, Chen
Dangdai Huagong Yanjiiu
Study and Control of Impurities in API
作者: Li Jianwen
52
项与 山东科源制药股份有限公司 相关的新闻(医药)2024-10-29
·赛柏蓝
编者按:本文来自米内网,作者桂枝;赛柏蓝授权转载,编辑相宜
数据显示,近年来咽喉中成药市场持续扩容,2023年在中国三大终端六大市场销售额接近70亿元,同比增长0.6%;2024年上半年销售额超过38亿元,同比增长12.08%。
中国三大终端六大市场咽喉中成药销售情况(单位:万元)
从三大终端上看,公立医院终端、公立基层医疗终端、零售药店终端咽喉中成药上半年销售额分别同比增长14.18%、13.95%、11.10%。其中,城市实体药店是咽喉中成药主要销售市场,2023年、2024年上半年分别占据63.25%、61.05%的市场份额。
目前咽喉中成药在售剂型主要有片剂、散剂/颗粒剂、丸剂、胶囊剂、吸入剂等。其中,片剂类咽喉中成药最为畅销,2023年、2024年上半年分别占据44.05%、42.68%的市场份额;散剂/颗粒剂销售额排名第二,占比19%;丸剂销售额排名第三,占比17.41%。
值得关注的是,目前国内布局咽喉中药新药的企业并不多,康恩贝的中药2.1类改良型新药清喉咽含片于2023年9月申报上市,贵州瑞和制药的中药1.1类新药喉喑清胶囊于2024年1月申报上市。
2022年至今,国内仅有无锡济煜山禾医药的中药2.1类改良型新药黄氏响声儿童喷雾剂、扬子江药业的中药1.1类新药芩翘咽舒颗粒、广州王老吉的中药2.3类改良型新药克感利咽口服液(新增适应症:急性咽炎)先后获批临床。
01
院内市场16个独家品种霸屏
华森、贵州三力上榜
2024年上半年中国公立医疗机构终端咽喉中成药一级集团销售额排名中,远大健康、湖南时代阳光药业、重庆华森制药、贵州三力制药、津药达仁堂集团依次位列前五。其中,多家企业销售额涨幅亮眼,5家企业市场份额合计接近70%。
2024H1中国公立医疗机构终端咽喉中成药销售额TOP5一级集团
咽喉中成药品牌TOP20中,重庆华森制药的甘桔冰梅片自2021年以来持续位列第一,湖南时代阳光药业的喉咽清口服液位列第二,贵州三力的开喉剑喷雾剂位列第三,津药达仁堂集团的清咽滴丸位列第四,西安碑林药业的金嗓散结胶囊位列第五,TOP5品牌上半年销售额均超过1亿元。
2024H1中国公立医疗机构终端咽喉中成药品牌TOP20
16个中成药为独家品种,包括重庆华森制药的甘桔冰梅片、贵州三力的开喉剑喷雾剂、津药达仁堂集团的清咽滴丸、无锡济煜山禾药业的黄氏响声丸、雷允上药业的六神胶囊、桂林三金药业的桂林西瓜霜含片、黄果树立爽药业的咽立爽口含滴丸等。
从企业层面上看,西安碑林药业有5个独家品种金嗓散结胶囊、金嗓利咽胶囊、金嗓散结颗粒、金嗓开音胶囊、金嗓清音胶囊上榜,湖南时代阳光药业有2个独家品种喉咽清口服液、喉咽清颗粒上榜,雷允上药业有2个品种六神丸、六神胶囊上榜。
14个品牌销售额涨逾10%,其中贵州三力的开喉剑喷雾剂大涨59.07%,雷允上药业的六神丸大涨46.79%,西安碑林药业的金嗓清音胶囊大涨42.82%,湖南时代阳光药业的喉咽清颗粒、广西金秀圣堂药业的双山颗粒均涨逾32%。
02
实体药店独家品种暴涨627%
桂林三金、达仁堂上榜
2024年上半年中国城市实体药店终端咽喉中成药一级集团格局中,TOP5一级集团分别为广西金嗓子、桂林三金集团、华润医药、桂龙药业、力诺集团。其中,广西金嗓子占据的市场份额达17.77%,桂林三金集团占比15.48%。
2023年中国城市实体药店终端咽喉中成药销售额TOP5一级集团
2024H1中国城市实体药店终端咽喉中成药品牌TOP20中,广西金嗓子的金嗓子喉片自2015年以来持续位列第一,以超过4亿元的销售额遥遥领先其他品牌;桂林三金药业的桂林西瓜霜、西瓜霜润喉片分别位列第二、第三,宏济堂的金鸣片位列第四,江中药业的复方草珊瑚含片位列第五,销售额均超过1亿元。
2024H1中国城市实体药店终端咽喉中成药品牌TOP20
从企业层面上看,桂林三金药业有3个独家品种桂林西瓜霜、西瓜霜润喉片、西瓜霜清咽含片上榜;华润医药旗下有2个品牌上榜,具体为江中药业的复方草珊瑚含片、华润三九的咽炎片;桂龙药业也有2个品牌上榜,包括清喉利咽颗粒、复方青橄榄利咽含片。
14个品牌销售额实现正增长,7个品种涨逾30%。其中,津药达仁堂集团的清咽滴丸暴涨627.17%、太极集团重庆中药二厂的玄麦甘桔颗粒大涨77.83%、石药欧意药业的复方熊胆薄荷含片大涨71.33%、上海医药青岛国风药业的苦金片大涨68.37%、片仔癀的复方片仔癀含片大涨51.82%。
桂林三金药业的西瓜霜润喉片为OTC乙类(双跨)品种,具有清音利咽、消肿止痛之功效,用于防治咽喉肿痛,声音嘶哑,喉痹,口舌生疮;急、慢性咽喉炎,急性扁桃体炎,口腔溃疡,口腔炎,牙龈肿痛。2020年至今该产品销售额持续呈现两位数增速,2023年、2024年上半年销售额均同比增逾11%。
津药达仁堂集团的独家品种清咽滴丸为国家医保甲类、国家基药目录、OTC甲类品种,具有疏风清热、解毒利咽之功效,用于风热喉痹,咽痛,咽干,口渴;或微恶风,发热,咽部红肿,急性咽炎见上述症候者。该产品2023年、2024年上半年销售额分别增长61.22%、627.17%,在咽喉中成药品牌TOP20中排名进一步上升至第12位。
资料来源:米内网数据库
END
内容沟通:郑瑶(13810174402)
左下角「关注账号」,右下角「在看」,防止失联
申请上市临床申请
2024-10-26
明日,2024医保谈判将正式开启为期4天的现场谈判/竞价。
10月24日,国家医保局官宣新一轮国谈即将开始。按照《2024年国家医保药品目录调整工作方案》,从7月1日正式启动国家医保局成立以来的第7次国家医保药品目录调整,经形式审查、专家评审、结果确认,共有162个通用名药品确认参加谈判/竞价。
在医保控费的大背景下,围绕支持有临床价值的创新的政策陆续发布,CDE在本周发布新规,提出新药等品种上市许可申请可享受理靠前服务,让创新药有了在审批中“插队”的机会。
资本市场上,随着9月“并购六条”的出台,10月份上市公司的并购潮开始,尤其是此前被严格限制的跨界并购又开始兴起波澜,本周仍在持续。
科源制药发布了《关联交易预案》,拟收购山东宏济堂制药99.42%股权;同期四川双马发布公告,公司拟使用自有及自筹资金购买其所持有的深圳市健元医药科技有限公司92.1745%的股权,交易实施完毕后,深圳健元成为公司合并报表范围内的控股子公司。
证监会再度鼓励上市公司兼并重组,用意或是在IPO收紧的大背景下,引导更多资源要素向新质生产力方向聚集,推动产业升级,支持创新。
大型药企方面,新一轮人事变动也在持续。本周,太极集团聘任了新总经理,东阿阿胶董事长白晓松辞职,华润博雅生物招了新任副总裁……
本周还有哪些大事发生?
政策动态
医保谈判对创新药支持成效显著,新一轮谈判即将开展:国家医保局官网显示,根据工作安排,2024年医保目录现场谈判/竞价拟于10月27日-30日在北京开展,预计11月份公布调整结果,新版药品目录将于2025年1月1日起实施。国家医保局成立以来,在坚持“保基本”的前提下,通过及时将创新药以合理价格纳入目录,并支持加快临床应用等方式,大力支持创新药发展。2023年通过谈判新增进入医保目录的105个药品,前三季度惠及797.8万人次,9月份药品销售额是1月份的7倍。6年来,谈判新增的446个药品,协议期内医保基金累计支出超3400亿元,惠及8亿人次,带动相关药品销售总额近5000亿元。统计显示,目前全国公立医院采购的药品中,目录内药品采购金额占比已超90%。
国家药监局表示将健全支持人工智能和医学影像医疗器械相应产业创新发展机制:10月25日,人工智能和医学影像医疗器械创新发展座谈会在北京召开。会议交流了人工智能和医学影像产品研发使用情况,聚焦创新发展共性问题,研讨支持政策。会议认为,人工智能和医学影像医疗器械是医疗器械领域新质生产力的代表,健全支持相应产业创新发展机制,是落实党的二十届三中全会精神和改革要求重要举措。下一步,国家药监局将着力研究破解产业发展“堵点”“难点” ,以问题为导向,强化部委间合作,进一步研究针对性举措,加快推动创新产品上市应用,促进相应产业创新高质量发展,更好满足公众用械需求。
11月起创新药等品种上市许可申请可享受理靠前服务:据国家药品监督管理局药品审评中心消息,为进一步鼓励药物研发创新,促进生物医药产业高质量发展,加快新药好药上市,经请示国家药品监督管理局同意,2024年11月1日起,对创新药以及经沟通交流确认可纳入优先审评审批程序和附条件批准程序的品种上市许可申请提供受理靠前服务。受理靠前服务主要解决创新药以及经沟通交流确认可纳入优先审评审批程序和附条件批准程序的品种在上市许可申请受理环节涉及的政策法规、申报程序以及证明性文件等问题,不包括技术审评相关问题。药品长三角分中心、药品大湾区分中心向区域内药品注册申请人提供受理靠前服务。
国家药监局与丹麦药品管理局签署合作意向书:10月24日,国家药监局局长李利与丹麦药品管理局代理局长梅特·艾伯·汉森在京签署了《中华人民共和国国家药品监督管理局与丹麦药品管理局合作意向书》,进一步加强双方在药品医疗器械监管领域交流合作。双方同意,共同推动中丹药品医疗器械战略领域二期合作项目,进一步在药品医疗器械监管领域开展务实合作。
全国中成药集采方案征求稿出炉:10月25日,湖北医保服务平台发出全国中成药联采办于公开征求《全国中成药采购联盟集中采购文件》(征求意见稿)和《全国中成药采购联盟集中采购文件(首批扩围接续)》 (征求意见稿)意见的通知,这意味着湖北牵头的第三批中成药集采正式官宣,即将开启全国集采。上述两个征求意见稿可于11月1日前将意见或建议进行反馈,与此前流出的报量目录相比,此次公布的品种范围完全一致,都是20个产品组95个品种。
云南省卫生健康委员会发布《云南省深化紧密型县域医疗卫生共同体建设若干措施》:10月22日,云南省卫生健康委员会发布《云南省深化紧密型县域医疗卫生共同体建设若干措施》,目的是持续巩固云南省紧密型县域医疗卫生共同体建设质效,到2027年底全省基本实现县域医共体全覆盖。具体措施包括:一是坚持政府主导,因地制宜组建县域医共体;二是健全运行机制,提高县域医共体管理质效;三是加强县域统筹,提高服务同质化水平;四是深化改革协同,完善县域医共体支持政策;五是强化监测评价,保障县域医共体健康运行。
天津市医疗保障局发布《就医费用报销“高效办成一件事”工作方案》:10月22日,天津市医疗保障局发布《就医费用报销“高效办成一件事”工作方案》,适用于天津市职工基本医疗保险和城乡居民基本医疗保险参保人员线上办理相关业务。重点任务包括:一是开设“一件事”办理专区;二是推进就医费用报销“一件事”;三是开发就医费用报销“一件事”服务功能;四是推进医保高频事项“跨省通办”“一网通办”。
大型制药
百时美施贵宝全球首创心肌肌球蛋白抑制剂迈凡妥(玛伐凯泰胶囊)在国内上市:10月24日,百时美施贵宝宣布,继获得中国国家药品监督管理局(NMPA)优先审评批准后,创新治疗药物迈凡妥(通用名:玛伐凯泰胶囊)现已在国内上市,用于治疗纽约心脏协会(NYHA)心功能分级II-III级的梗阻性肥厚型心肌病(HCM)成人患者,以改善运动能力和症状。这是全球首创,也是目前唯一获批用于治疗梗阻性肥厚型心肌病的心肌肌球蛋白抑制剂。
人福医药司美格鲁肽注射液获得药物临床试验批准通知书:人福医药10月25日晚间公告,公司控股子公司宜昌人福近日收到国家药品监督管理局核准签发的司美格鲁肽注射液的药物临床试验批准通知书。司美格鲁肽是一种长效人胰高血糖素样肽-1(GLP-1)受体激动剂,能够刺激胰岛素的生成,并抑制胰高血糖素分泌,同时降低食欲和食物摄入量,从而在治疗2型糖尿病和辅助体重管理方面发挥重要作用。
罗氏10.5亿美元押注CNS基因疗法:10月24日,应用人工智能(AI)实现体内基因递送的基因技术公司Dyno Therapeutics宣布与罗氏达成第二次研究合作,开发下一代腺相关病毒(AAV)载体,用于针对神经疾病的基因治疗。根据新协议的条款,罗氏将向Dyno预付5000万美元,以及在合作研究阶段的额外付款,加上潜在的临床前、临床和销售里程碑付款,总计超过10亿美元,以及产品净销售额的特许权使用费。
太极集团高层人事变动:10月24日,太极集团公告,公司董事会同意选举俞敏为公司董事长,同时,俞敏不再担任公司总经理职务。经公司董事会提名委员会资格审查,于宗斌符合担任公司高级管理人员任职要求,同意聘任于宗斌为公司总经理。
葵花药业拟投资1000万元设立全资子公司:10月24日,葵花药业发布公告,全资子公司葵花药业集团医药有限公司拟使用自有资金1000万元投资设立全资子公司葵花大药房(湖北)有限公司(筹)。葵花药业称,公司本次出资设立葵花大药房,旨在顺应行业发展趋势,打造线上自营平台,做强新渠道、影响新人群、拓展新增量,构建线上营销业务闭环管理。本次对外投资不涉及关联交易,不构成重大资产重组。
默沙东13亿美元开展新收购:10月23日,Modifi Biosciences宣布已与默沙东(MSD)达成协议。默沙东将通过子公司以3000万美元的预付款收购了Modifi Bio的所有已发行股份。Modifi Bio股东也有资格获得总额高达13亿美元的潜在里程碑付款。通过此次收购,默沙东将获得其创新癌症疗法。Modifi Bio的创新疗法是一种利用DNA修复缺陷治疗难治性癌症的临床前化合物,不靶向特定蛋白,而是直接靶向细胞的DNA。
东阿阿胶董事长白晓松辞职:10月22日,东阿阿胶发布公告,公司董事会近日收到白晓松先生、邓蓉女士提交的书面辞职报告,由于工作变动原因,白晓松先生申请辞去公司第十一届董事会董事长、董事职务,同时一并辞去第十一届董事会战略委员会主任委员职务;邓蓉女士申请辞去公司第十一届董事会董事职务,同时一并辞去第十一届董事会战略委员会、薪酬与考核委员会以及审计委员会委员职务。辞职后,白晓松先生、邓蓉女士不再担任公司及公司控股子公司任何职务;其中,白晓松先生将继续担任华润医药集团有限公司董事会主席,邓蓉女士将继续担任华润医药集团有限公司首席财务官。
华润博雅生物聘任新任副总裁:博雅生物于10月23日召开第八届董事会第六次会议,审议通过《关于聘任高级管理人员的议案》。根据相关规定,经公司总裁梁小明先生提名,聘任任辉先生担任公司副总裁,任期自本次董事会审议通过之日起至本届董事会届满之日止。任辉先生的任职资格已经公司董事会提名、薪酬与考核委员会审查,符合公司高级管理人员副总裁的任职资格。截至本公告披露日,任辉先生未直接或间接持有公司股份,未受过中国证监会及其他相关部门的处罚和证券交易所惩戒,不属于失信被执行人,也不存在相关禁止担任上市公司监事的情形,符合任职条件。
远大医药首款国产可调节颅内取栓支架产品获药监局批准上市:10月22日,港股科技创新型医药企业远大医药发布公告称,其用于治疗急性缺血性卒中的可调节颅内取栓支架产品“鸬鹚”日前已获药监局颁发医疗器械注册证书。鸬鹚是我国首款国产的可调节颅内取栓支架产品,此次产品获批是远大医药在神经介入板块产品布局的又一突破性进展。鸬鹚是首款国产的可调节颅内取栓支架产品。该款支架采用圆丝编织结构设计,可在体外进行手动调控至理想直径以匹配目标血管,同时支架植入过程中全程可视、全程显影,能够更好的辅助术者根据血栓部位与总长度,来调节支架以更好的适应闭塞血管,实现更高的血管再通率。
生物技术
荣昌生物泰它西普第三项适应症申报上市:10月26日,中国国家药监局药品审评中心(CDE)官网最新公示,荣昌生物研发的BLyS/APRIL双靶点融合蛋白创新药注射用泰它西普新适应症上市申请已获得受理。今年8月,荣昌生物发布新闻稿称,泰它西普用于治疗全身型重症肌无力(gMG)的中国3期临床研究达到临床试验主要研究终点。由此推测,本次该产品申报上市的适应症为可能为治疗全身型重症肌无力(gMG)。
睿昂基因多位高管被刑事拘留,涉嫌罪名变更为诈骗罪:10月25日晚,睿昂基因发布关于公司重大事项进展的公告。公告称,前期,公司实际控制人、董事长兼总经理、核心技术人员熊慧,实际控制人、董事兼副总经理熊钧,副总经理何俊彦,副总经理薛愉玮因涉嫌非法经营罪被公安机关采取指定居所监视居住等强制措施。近日,收到以上人员家属通知,熊慧、熊钧、薛愉玮、何俊彦的强制措施变更为刑事拘留,涉嫌罪名变更为诈骗罪。相关事项尚待进一步调查。睿昂基因表示,目前,公司已针对相关事项做出妥善安排,代理董事长,其他高管及核心技术人员正常履职,公司业务正常开展,经营活动正常进行。公司将持续关注上述事项的进展情况,并严格按照有关法律、法规的规定和要求,及时履行信息披露义务。敬请广大投资者理性投资,并注意投资风险。
百济神州回应大中华区首席商务官殷敏被带走调查:近日,百济神州大中华区首席商务官殷敏涉嫌阿斯利康“骗保”事件被带走调查,引发公司股价波动,10月25日午后,百济神州股价快速跳水,一度跌超10%,随后反弹拉升。殷敏于2022年1月正式加入百济神州,任大中华区首席商务官至今。10月25日,百济神州发布声明称,根据目前了解的情况,该员工所涉事件与百济神州无关。
亚虹医药自主研发的APL-2302临床试验申请获得FDA批准:近日,亚虹医药发布公告,公司收到美国食品药品监督管理局关于同意开展公司自主研发的APL-2302用于治疗晚期实体瘤临床试验的函告。据悉,APL-2302是一种泛素特异性蛋白酶1(USP1)抑制剂,通过“合成致死”机制发挥抗肿瘤作用。临床前研究提示APL-2302单药治疗和联合治疗在肿瘤抑制方面表现出良好的体外和体内活性。APL-2302有潜力成为治疗特定生物标志物阳性(如BRCA基因突变等)的晚期实体瘤(乳腺癌、卵巢癌、前列腺癌等)的新选择。
甘李药业GLR1023注射液中国Ⅰ期临床试验完成首例受试者给药:甘李药业日前公告,公司研发的生物类似药GLR1023注射液(司库奇尤单抗)完成了适应症为成人中度至重度斑块状银屑病的一项在中国健康受试者中的I期临床研究的首例受试者给药。银屑病是一种免疫相关的慢性、复发性、炎症性、系统性疾病,司库奇尤单抗作为治疗银屑病(牛皮癣)的生物制剂,其原研药是由诺华制药公司生产的一款全人源重组单克隆IgG1κ抗体。
资本市场
恒瑞医药回应赴港上市传闻:据报道,恒瑞医药正考虑在香港进行第二上市,最快可能明年进行。知情人士称,该公司正在与顾问机构商讨潜在的股票发行,可能筹集至少20亿美元(约156亿港元)。事项仍在考虑之中,融资规模将取决于市场状况。10月24日,就该市场传言,恒瑞医药发布公告回应称,“近年来,在科技创新和国际化发展战略驱动下,公司稳步推进国际化进程。为进一步深化公司战略发展目标,公司近期对境外资本市场融资等事项开展了研究咨询等前期工作。截至目前,公司就相关事项尚未确定具体方案。”同日,恒瑞医药公布2024年第三季度报告,今年前三季度,恒瑞医药实现营业收入201.89亿元,同比增长18.67%,归属于上市公司股东的净利润46.2亿元,同比增长32.98%。
诺泰生物被证监会立案调查:诺泰生物近日公告,公司及实际控制人之一赵德中于近日收到中国证券监督管理委员会《立案告知书》。根据《立案告知书》,因涉嫌信息披露违法违规,中国证监会决定立案。经诺泰生物自查,本次立案可能涉及公司2021年度技术转让相关事宜。公告显示,截至目前尚未收到中国证监会的最终调查结论,立案调查事项的最终结果将以中国证监会出具的结论为准。公司将积极配合中国证监会的相关工作,并严格按照监管要求履行信息披露义务。
科源制药收购宏济堂:10月22日,科源制药发布公告,董事会审议通过了发行股份及支付现金购买资产并募集配套资金暨关联交易预案的方案,公司计划通过发行股份及支付现金的方式,购买力诺投资控股集团有限公司、力诺集团股份有限公司等39名交易对方合计持有的宏济堂99.42%股权,并募集配套资金,交易完成后,宏济堂将成为科源制药的控股子公司。
水泥大佬收购减肥药原料药龙头:10月21日,四川和谐双马股份有限公司发布公告,公司拟使用自有及自筹资金以总计15.96亿元的交易价格向深圳市星银医药有限公司及深圳市星银投资集团有限公司购买其所持有的深圳市健元医药科技有限公司92.1745%的股权,其中,公司拟以13.6亿元购买星银医药所持有的深圳健元78.5458%的股权;以2.36亿元购买星银集团所持有的深圳健元13.6287%的股权。交易完成后,公司持有深圳健元92.1745%的股权,星银集团持有深圳健元3%的股权,自然人姚志勇持有深圳健元4.8255%的股权。本次交易实施完毕后,深圳健元成为公司合并报表范围内的控股子公司。
一审| 黄佳
二审| 李芳晨
三审| 李静芝
精彩推荐
CM10 | 集采 | 国谈 | 医保动态 | 药审 | 人才 | 薪资 | 榜单 | CAR-T | PD-1 | mRNA | 单抗 | 商业化 | 国际化 | 猎药人系列专题 | 出海
启思会 | 声音·责任 | 创百汇 | E药经理人理事会 | 微解药直播 | 大国新药 | 营销硬观点 | 投资人去哪儿 | 分析师看赛道 | 药事每周谈 | 医药界·E药经理人 | 中国医药手册
创新100强榜单 | 恒瑞 | 中国生物制药 | 百济 | 石药 | 信达 | 君实 | 复宏汉霖 |翰森 | 康方生物 | 上海医药 | 和黄医药 | 东阳光药 | 荣昌 | 亚盛医药 | 齐鲁制药 | 康宁杰瑞 | 贝达药业 | 微芯生物 | 复星医药 |再鼎医药|亚虹医药
跨国药企50强榜单 | 辉瑞 | 艾伯维 | 诺华 | 强生 | 罗氏 | BMS | 默克 | 赛诺菲 | AZ | GSK | 武田 | 吉利德科学 | 礼来 | 安进 | 诺和诺德 | 拜耳 | 莫德纳 | BI | 晖致 | 再生元
并购高管变更IPO
2024-10-26
·米内网
看点
view
本周,2024年国谈时间公布,第三批全国中成药集采官宣,医保资金监管持续强化;药品市场迎变局,首批创新药械目录出炉,58个新药新增进院,10个药品遭重点监控;研发新进展,正大天晴1类新药爆发,齐鲁进攻$3亿品种首仿;企业大新闻,中药巨头高层“大换血”,多家药企实控人被立案,百年中华老字号将被收购……医药行业一周大事件,精彩不容错过!
米内一周TOP榜
米内数据
集采冲击,造影剂明星药跌出TOP10
32个肝病中成药热销!独家注射剂医院封王
34款咽喉中成药厉害了!独家品种暴涨627%
糖尿病药王易主,明星药暴涨142%
药企动态
片仔癀净利润首破25亿,华海、佐力创新高
太极集团拿下7亿大品种
22亿大品种,浙江药企再下一城
南京正大天晴进军30亿市场
石四药两大新品同日获批
11月公布结果!162个药品参加医保谈判/竞价
10月24日,国家医保局官微发布《医保谈判对创新药支持成效显著,新一轮谈判即将开展》的文章,其中提到,经形式审查、专家评审、结果确认,共有162个通用名药品确认参加谈判/竞价。根据工作安排,2024年医保目录现场谈判/竞价拟于10月27日-30日在北京开展,预计11月份公布调整结果,新版药品目录将于2025年1月1日起实施。(国家医保局)
日均费用+降幅决定中选
10月25日,湖北医保服务平台发布通知,就《全国中成药采购联盟集中采购文件》(征求意见稿)和《全国中成药采购联盟集中采购文件(首批扩围接续)》 (征求意见稿)公开征求意见,这意味着湖北牵头的第三批全国中成药集采正式官宣启动。与此前流出的报量目录相比,此次公布的品种范围完全一致,都是20个产品组95个品种。(医药云端)
划重点:
1、申报价为申报企业的实际供应价,申报价不高于基准价格和省级、省际联盟集采中选价格的,为有效报价;2、同采购组内分为A、B两个竞争单元,分别竞争;3、以综合得分由高到低排序,确定入围企业,综合得分=价格竞争得分×60%+技术评价得分×40%。
强化监管!今年1-9月追回医保资金160亿元
10月22日,2024年医保基金监管趋势交流会暨蓝皮书发布会召开。据悉,截至9月,国家飞检已覆盖全国所有省份,检查定点医药机构500家,查出涉嫌违规金额22.1亿元。其中,根据大数据模型线索,开展了专项飞检的定点医药机构达185家,查实欺诈骗保机构111家。今年1-9月,全国各级医保部门共追回医保资金160.6亿元。(中国青年报)
24个产品!首批创新药械目录出炉
10月21日,广州市工信局发布通知,公布《广州市创新药械产品目录》首批入选产品,共有7个药品、17个医疗器械在列,覆盖肿瘤、免疫及心血管等重大疾病领域。据介绍,纳入目录的创新药械有望获得广州市在药品研究开发、医院准入、医院处方/使用、药品支付等全链条的重点支持。(广州市工信局)
延伸阅读:
7个入选药品分别为:百奥泰的贝伐珠单抗注射液、托珠单抗注射液、阿达木单抗注射液3款生物类似药,以及1类新药枸橼酸倍维巴肽注射液;此外,还有康方的卡度尼利单抗注射液,一品红的苯磺酸氨氯地平干混悬剂、百济神州的替雷利珠单抗注射液。
58个品种新增进院!5个超10亿独家中成药在列
10月21日,四川大学华西医院发布《新药引进公告》,该院药事管理委员会严格按照相关规定与流程,对前期申报进院的药品品种进行了认真审议与讨论,最终决定引进58个品种,包括46个西药、12个中成药。从企业看,恒瑞医药以4个品种领跑,拜耳、费森尤斯卡比、科伦药业、诺华、云南白药、正大天晴药业均有2个品种在列。(米内网整理)
米内数据:
12个中成药中有11个为独家品种(含独家剂型),其中以岭药业的通心络胶囊、贵州健兴药业的肺力咳合剂、云南白药的云南白药气雾剂、太极集团的急支糖浆、云南白药的云南白药膏2023年在中国三大终端六大市场(统计范围详见文末)的销售额均超10亿元。
10个品种在列!重点监控药品名单更新了
10月21日,上海阳光医药采购网发布通知,公布2024年8月药品挂网公开议价采购监管品种名单,“未通过公允性品种”和“重点监控品种”各有10个品种被纳入。经统计,今年以来,上海已公布了6批药品挂网公开议价采购监管品种名单,共有16个药品(以药品名+企业计)被纳入重点监控,其中有9个为注射剂。(米内网整理)
延伸阅读:
公布品种仅作为提醒,要求各医疗机构根据市场供需情况以及阳光平台“红黄绿线”议价实时提醒信息合理议价,既保证临床需求,又避免不合理调价,采购质量可靠、价格适宜的药品。
101个药品暂停挂网,44个药品遭处置
10月24日,陕西省公共资源交易中心发布通知,对属于第一至七批国家集采部分非供应省份药品,在该省备选目录内的产品暂停挂网,共涉及101个药品(以药品统一编码计,下同);10月22日,陕西省公布该省第一至七批国家集采药品非供应省份挂网价高于集采中选价1.5倍且高于网络药店价格1.5倍以上未按9月24日通知要求降价的产品的处置结果,其中23个药品被列为黄标,21个药品被列为红标。(陕西省公共资源交易中心)
国产首家报产!齐鲁制药进攻$3亿品种
10月23日,CDE官网显示,齐鲁制药的4类仿制药甲磺酸贝舒地尔片提交上市申请获受理,为该产品国内首个报产的仿制药。贝舒地尔是赛诺菲开发的一款靶向Rho激酶抑制剂,2023年全球销售额超过3亿美元。该新药于2023年8月获批进入国内市场,是中国首个且唯一获批的治疗慢性移植物抗宿主病(cGVHD)的靶向药物。(米内网整理)
今年以来29款!正大天晴1类新药大爆发
10月23日,正大制药官微发文,其下属企业正大天晴药业的2款1类新药——注射用TQB2029、TQB3002片接连首次获批临床。其中,注射用TQB2029是一款靶向GPRC5D和CD3的双抗,拟开发用于治疗多发性骨髓瘤,而TQB3002片是一款四代EGFR抑制剂,拟开发治疗晚期恶性肿瘤。今年以来,正大天晴药业有29款1类新药获批临床(含非首次获批,不含补充申请),其中抗肿瘤和免疫调节剂占比超八成。(米内网整理)
米内数据:
随着越来越多的创新药获批上市并实现销售,叠加临床刚需属性拉动,近年来中国三大终端六大市场抗肿瘤和免疫调节剂(化学药+生物药)销售额逐渐上涨,2023年超过2200亿元,2024上半年以约7.5%的增速继续攀升。
华海、华东......上榜2024年浙江省民营经济总部领军企业
10月22日,浙江省经济和信息化厅发布新闻,公布2024年浙江省民营经济总部领军企业名单,共有117家企业上榜,包括华东医药、浙江医药、华海药业等多家药企。(浙江省经济和信息化厅)
涉嫌信披违法违规!多家药企实控人被立案调查
本周,多家上市药企实控人被立案。10月23日晚间,诺泰生物公告称,因涉嫌信息披露违法违规,公司及实控人之一赵德中被立案。经公司自查,本次立案可能涉及公司2021年度技术转让相关事宜;10月21日晚间,四环生物公告称,公司实控人陆克平因涉嫌信息披露违法违规,中国证监会决定对其立案。(米内网整理)
百年中华老字号将被收购
10月22日,山东科源制药公告称,公司正在筹划通过发行股份及支付现金购买力诺投资控股集团、力诺集团等39名交易对方合计持有的宏济堂99.42%股权并募集配套资金。据悉,宏济堂是一家百年中华老字号企业,主营业务为中成药、阿胶制品及麝香酮等产品的研发、生产与销售,目前共取得150个药品注册批件。(证券日报)
业内观点:
科源制药收购宏济堂,是一场中药与化药资产的整合,将有机会借政策的“东风”快速发展。
8人辞职!中药巨头高层“大换血”
10月23日,东阿阿胶连发多份公告,宣布了一系列人事变动,包括董事长、总裁、监事会主席等8人辞职的消息,除程杰就任董事长之外,其余7人不再担任东阿阿胶及控股子公司任何职务。(医药代表)
注:米内网《中国三大终端六大市场药品竞争格局》,统计范围是:城市公立医院和县级公立医院、城市社区中心和乡镇卫生院、城市实体药店和网上药店,不含民营医院、私人诊所、村卫生室,不含县乡村药店;上述销售额以产品在终端的平均零售价计算。投稿及报料请发邮件到872470254@qq.com稿件要求详询米内微信首页菜单栏商务及内容合作可联系QQ:412539092
【分享、点赞、在看】点一点不失联哦
带量采购一致性评价
100 项与 山东科源制药股份有限公司 相关的药物交易
登录后查看更多信息
100 项与 山东科源制药股份有限公司 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
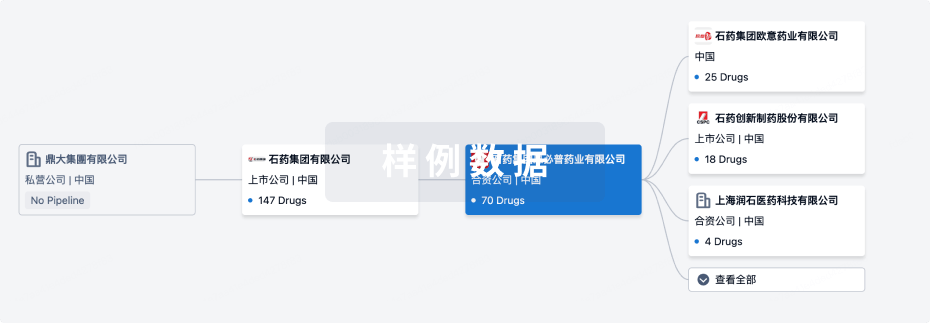
管线布局
2024年11月17日管线快照
管线布局中药物为当前组织机构及其子机构作为药物机构进行统计,早期临床1期并入临床1期,临床1/2期并入临床2期,临床2/3期并入临床3期
批准上市
2
登录后查看更多信息
当前项目
| 药物(靶点) | 适应症 | 全球最高研发状态 |
|---|---|---|
富马酸氯马斯汀 ( H1 receptor ) | 湿疹 更多 | 批准上市 |
氨来呫诺 ( IKKε x TBK1 ) | 溃疡 更多 | 批准上市 |
登录后查看更多信息
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

转化医学
使用我们的转化医学数据加速您的研究。
登录
或
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

