预约演示
更新于:2025-06-06

Hansoh Pharmaceutical Group Co., Ltd.
更新于:2025-06-06
概览
关联
100 项与 翰森製藥集團有限公司 相关的临床结果
登录后查看更多信息
0 项与 翰森製藥集團有限公司 相关的专利(医药)
登录后查看更多信息
4
项与 翰森製藥集團有限公司 相关的文献(医药)2025-06-03·Journal of Translational Medicine
Correction: Overview of preclinical and phase II clinical studies on Pegmolesatide's long-term erythropoiesis stimulating effect via EPOR-mediated signal transduction.
作者: Yu, Xueqing ; Mei, Changlin ; Yu, Hai ; Luo, Weili ; Tao, Ye ; Li, Zhen ; Ma, Xiaoying ; Zhang, Lu ; Hu, Zhizhen ; Zhou, Yuanfeng ; Li, Yunfan ; Qian, Hui ; Li, Ping ; Wang, Dandan ; Chen, Qinkai
2025-03-01·Kidney International Reports
Randomized Trial of Pegmolesatide for the Treatment of Anemia in Patients With Nondialysis CKD
Article
作者: Tang, Shuifu ; Yu, Xueqing ; Zhong, Aimin ; Wang, Fan ; Qiu, Hongyu ; Xie, Jianteng ; Li, Chuan ; Ma, Anran ; Huang, Xiangyang ; Yang, Aicheng ; Jia, Xiaohong ; Peng, Xiaomei ; Chen, Qinkai ; Lu, Wanhong ; He, Liangliang ; Wang, Qin
Introduction:
Pegmolesatide has been recently approved for treating anemia in chronic kidney disease (CKD) patients in China. We presented the results of the pivotal study conducted in patients with nondialysis-dependent (NDD)-CKD with anemia.
Methods:
This randomized, active-controlled, open-label, noninferiority phase 3 study was conducted across 38 centers in China. Eligible patients were randomly assigned to receive subcutaneous injection of pegmolesatide in the upper arm once every 4 weeks or epoetin alfa weekly or biweekly, with doses adjusted to maintain hemoglobin (Hb) level of 100 to 120 g/l. The primary outcome was the mean change in Hb level from the baseline during the efficacy evaluation period. Noninferiority of pegmolesatide to epoetin alfa was established if the lower limit of the 2-sided 95% confidence interval (CI) was ≥ -10 g/l.
Results:
A total of 173 patients received at least 1 dose of pegmolesatide (115 patients) or epoetin alfa (58 patients). During the efficacy evaluation period, the mean change in Hb from baseline level was 19.2 g/l in the pegmolesatide group and 15.4 g/l in the epoetin alfa group with a between-group difference of 3.8 g/l (95% CI: 0.7-6.9). The incidence of adverse events (AEs) and serious AEs (SAEs) were similar between groups, with hypertension being the most reported AE related to the study drug. No drug-related hypersensitivity reactions or fatal events were observed.
Conclusion:
Pegmolesatide demonstrated comparable efficacy to epoetin alfa in elevating and maintaining Hb levels in patients with NDD-CKD with anemia without new safety concerns (ClinicalTrials.gov identifier: NCT03903809).
2024-06-28·CHEMICAL & PHARMACEUTICAL BULLETIN
Systematic DOE Approach for Dry Granulation Study at Early Clinical Stage of Novel Drugs: An Industrial Case
Article
作者: Dong, Zhiying ; Yuan, Hengli ; Shen, Jinyang ; Chen, Weiqi ; Zhang, Shicheng ; Wang, Chun ; Yao, Jianping ; Wang, Xiaolei ; Guan, Qixuan
In order to introduce a cost-effective strategy method for commercial scale dry granulation at the early clinical stage of drug product development, we developed dry granulation process using formulation without API, fitted and optimized the process parameters adopted Design of Experiment (DOE). Then, the process parameters were confirmed using one formulation containing active pharmaceutical ingredient (API). The results showed that the roller pressure had significant effect on particle ratio (retained up to #60 mesh screen), bulk density and tapped density. The roller gap had significant influence on particle ratio and specific energy. The particle ratio was significantly affected by the mill speed (second level). The tabletability of the powder decreased after dry granulation. The effect of magnesium stearate on the tabletability was significant. In the process validation study, the properties of the prepared granules met the requirements for each response studied in the DOE. The prepared tablets showed higher tensile strength, good content uniformity of filled capsules, and the dissolution profiles of which were consistent with that of clinical products. This drug product process development and research strategies could be used as a preliminary experiment for the dry granulation process in the early clinical stage.
1,557
项与 翰森製藥集團有限公司 相关的新闻(医药)2025-06-05
曾抄底数笔中国创新资产的BioNTech,在与BMS的合作中,以“中间商”的角色狠狠赚了巨额差价。这背后带来的认知是:我们仍然没有定价权。撰文|Erin10亿美元收购普米斯,转手111亿美元把普米斯的双抗又卖给BMS,行业为这手“高回报”的倒买倒卖惊呼的同时,也对BioNTech——这家曾经的mRNA明星质疑声四起,一边低位抄底,一边高价出手,一时间,“中间商赚差价”“二道贩子”的标签不胫而走。这家曾一度被视为mRNA平台代表的技术先锋,如今为何成了资本套利的代名词?而透过这场“天价”转手交易所掀起的波澜,或许能更清晰地看见中国Biotech在全球产业链中的真实位置。中国青苗“抄底王”在转手BNT327给BMS的重磅交易之后,BioNTech迅速成为行业舆论的中心。一边是创下双抗领域估值纪录的111亿美元,一边是曾以10亿美元“低价”收购普米斯的“先手操作”。这种近乎10倍的转手操作,让行业开始重新审视这家mRNA明星公司的角色定位:它是那个mRNA技术先驱的Biotech,还是正逐步演变为转卖抄底资产的“二道贩子”?事实上,BioNTech真正进入中国市场,并不是在这笔“天价转手”之后才引发关注。早在2023年,国内Biotech处于“至暗时刻”:IPO停滞、融资收紧、估值跳水,“找钱”与“活下去”成为当年最频繁的行业关键词。而BD交易几乎成为Biotech们最后一根“救命稻草”。也正是在这个节点,BioNTech开启了其对中国市场的密集扫货,几乎成了中国“青苗”管线最大买家之一。仅仅一年内,就先后与普米斯、宜联、道尔、映恩、昂科免疫等5家中国Biotech达成6笔交易,布局抗体、ADC、双抗等多个领域。来源:公开信息整理并且不同于传统MNC注重中后期产品和全球商业化潜力的投资偏好,BioNTech的BD风格独树一帜:所有项目几乎都处在临床前或早期阶段;聚焦适配自身mRNA平台或肿瘤管线的协同;合作不仅包括候选药物本身,还频繁“捆绑”CMC、CDMO乃至中国区临床执行资源。有意思的是,从今天回看,其“眼光”也的确精准:CTLA-4抗体、HER2 ADC、Trop2 ADC、HER3 ADC、PD-L1/VEGF双抗……均是这两年全球范围内竞争最为激烈的热门靶点。而其中最具代表性的,当属来自普米斯的PD-L1/VEGF双抗新药PM8002(BNT327)。2023年10月,BioNTech以5500万美元首付款、总额不超过10亿美元的交易条件拿下这款PD-L1/VEGF双抗的全球权益。彼时,PM8002在行业内尚属“边缘角色”,临床数据也未形成明确差异化价值。但仅一年之后,情况完全逆转。康方生物公布依沃西“头对头”打败K药的消息,使得市场情绪瞬间点燃。而BNT327因靶点、结构与依沃西类似,其价值迅速被重估。惊觉“淘到好货”的BioNTech,在2024年11月果断出手,以8亿美元+1.5亿美元里程碑收购普米斯全部股权,将这颗“潜力种子”彻底收入囊中,并且提速做研发,围绕该产品密集开展了多个注册临床。正如BioNTech所料,仅约半年的时间,BMS就扛着巨款找上了门,宣布与BioNTech达成合作。根据合作条款,BMS将向BioNTech支付15亿美元首付款,并在2028年前支付总计20亿美元。此外,BioNTech将有资格获得高达76亿美元的里程碑付款。总体加起来,这笔潜在交易总额有望达到111亿美元。这场价格高出10倍的“倒手”让市场舆论哗然,纷纷感叹普米斯“卖亏了”的同时,行业的另一面是:BioNTech从mRNA明星瞬间“失格”,成了靠包装资产、吃差价的“中间商”。纵观BioNTech手里的创新资产,有多笔来自中国,不知道类似的“吃”差价行为,还会不会继续上演。 推荐阅读 5年现金流翻近百倍,从合作MNC到成为MNC,BioNTech如何稳度周期?四次“扫货”中国Biotech又为何?1000亿魔幻“赌局”:中国双抗“完”了?“二道贩子”还是押宝高手?不过,如果仅将BioNTech解读为一位“抄底套利”的买手,恐怕仍显肤浅。实际上,自2020年因mRNA疫苗名声大噪之后,BioNTech就被推至全球生物科技聚光灯下,其强平台背景、创始团队技术底色及庞大现金储备为后续转型打下了基础。这家由科学家创立的公司,其实创立之初就在强调“聚焦肿瘤mRNA个体化疫苗”。即便新冠疫苗带来爆炸性增长,该公司创始人也反复在公开场合强调:“我们始终是一家癌症免疫治疗公司。”值得一提的是,BioNTech曾公开表示要在2026年推出第一款抗肿瘤产品。因此在2023年其实对于BioNTech来说,也是摆脱mRNA标签,重回抗肿瘤路线的转折点。既要维持mRNA等底层创新平台的更新,又需在中短期内打造多条成药性强的抗肿瘤产品线,同时保持内部临床推进能力。也就是说,对于BioNTech来说,BD或许不是随机动作,而更像是填补平台与商业转化间“断层”的过渡措施。在其年报中,BioNTech也指出,mRNA之外,将围绕抗体药物、TCR-T、ADC等多个技术方向展开组合型投入,以“系统性拓展肿瘤产品组合”为目标。对中国Biotech的大量“抄底”,正是这轮转折的一部分。并且在过去几年中,BioNTech已围绕肿瘤免疫、抗体工程及细胞治疗三大方向在全球完成多个关键交易:与Genmab合作,基于HexaBody平台开发多靶点抗体;与Matinas合作推进新型mRNA递送系统,布局平台替代方向。与AI药物设计企业InstaDeep达成战略整合,2023年正式完成全资收购,建立AI辅助研发的内生能力;与TCR-T疗法公司Medigene达成超过10亿美元潜在价值的研发合作,用于拓展固态肿瘤免疫治疗产品。在内部研发方面,截至2024年末,BioNTech肿瘤管线已拓展至30项临床资产,其中20余项进入全球多中心试验阶段,尤其强化了与mRNA疫苗的组合开发能力。不过,从BioNTech官网最新披露的信息来看,其围绕抗肿瘤已经建立了mRNA和抗体治疗两大条线,其中抗体治疗的临床管线几乎全部来自于在中国的BD和收购。而从BNT327的交易细节来看,BioNTech也并不是“空手套白狼”。一方面,管线结构调整后,BioNTech为BNT327开展了至少10项适应证、20项临床试验,全球入组人数过千;另一方面,BNT327的开发成本由BioNTech先期承担,并系统性推进多个ADC联合治疗策略;此外,与BMS交易中,设置了明确的“15亿美元首付款+20亿美元无条件里程碑+五五分摊后续投入”条款,大量回报依赖于未来的商业化兑现,而不是“一锤子买卖”。也就是说,即便首付款看上去惊人,未来的不确定性与分摊责任,依然对BioNTech构成不小挑战。若最终临床失败或商业不达预期,损失也将由其共同承担。回到国内视角,这场“BioNTech争议”之所以引发如此多讨论,本质上反映的是中国Biotech在全球创新价值链中所处的角色焦虑。当国内创新药尚不具备全链条全球布局能力时,价值实现的通道只剩“被收购、被BD”;而在资金、临床试验体系与商业化能力的缺位,使得真正有价值的资产,往往只能以折价换取现金流。如果更多中国Biotech不仅能做出好管线,还能掌控海外临床布局、资本调度与BD谈判——也就是掌握“议价话语权”时,“中间商赚差价”或也将不再成立,BioNTech们也将失去用低价“套取”高估值的空间。恰如一位业内人士告诉E药经理人:“中国药企要避免中间商差价的终极方案,即自主海外商业化,畅销全球,成为China-based MNC。”当然,这其中的核心还是定价权。一审| 黄佳二审| 李芳晨三审| 李静芝精彩推荐大事件 | IPO | 融资&交易 | 财报季 | 新产品 | 研发日 | 里程碑 | 行业观察 | 政策解读 | 深度案例 | 大咖履新 | 集采&国谈 | 出海 | 高端访谈 | 技术&赛道 | E企谈 | 新药生命周期 | 市值 | 新药上市 | 商业价值 | 医疗器械 | IND | 周年庆大药企 | 竞争力20强 | 恒瑞 | 石药 | 中生制药 | 齐鲁 | 复星 | 科伦 | 翰森 | 华润 | 国药 | 云南白药 | 天士力 | 华东 | 上药创新药企 | 创新100强 | 百济 | 信达 | 君实 | 复宏汉霖 | 康方 | 和黄 | 荣昌 | 亚盛|康宁杰瑞|贝达|微芯|再鼎|亚虹跨国药企|MNC卓越|辉瑞|AZ|诺华|罗氏|BMS|默克|赛诺菲|GSK|武田|礼来|诺和诺德|拜耳供应链|赛默飞|药明|凯莱英|泰格|思拓凡|康龙化成|博腾|晶泰|龙沙|三星启思会 | 声音·责任 | 创百汇 | E药经理人理事会 | 微解药直播 | 大国新药 | 营销硬观点 | 投资人去哪儿 | 分析师看赛道 | 药事每周谈 | 中国医药手册
信使RNA抗体药物偶联物并购IPO
2025-06-05
·深蓝观
吴妮 | 撰文旧梦 | 编辑最近,PD-(L)1/VEGF双抗赚足了热度。自康方用一个头对头K药的试验彻底打开这一赛道之后,就不断有新的大小玩家竞相涌入。而最近的两个大额deal也是把这个古早又新兴的治疗Class推向高潮。三生制药PD-1/VEGF双抗SSGJ-707的全球权益授权给辉瑞,潜在总金额高达60.5亿美元,其中首付款达到12.5亿美元,刷新了国产创新药对外授权的最高首付款纪录。普米斯研发的PD-L1/VEGF双抗PM8002被BioNTech收购后,如今转手BMS,身价翻5倍。BioNTech将获得15亿美元的首付款,以及高达76亿美元的潜在额外开发、监管和商业里程碑付款。赛道很火热,遗憾的是后者。在旁观者眼中:这种“中国低价卖、‘二道贩子’转手翻倍再卖出”的戏码再一次上演:PD-L1/VEGF双抗体早在2023年11月授权给BioNTech,当时已经处于二期临床阶段,仅以5500万美元首付款和十多亿美元里程碑付款就license out中国大区以外权益。虽然这次BMS同BioNTech交易时,该药已进入三期临床阶段,需要重新估量身价。但4-5倍的价格差,还是不免有些令人唏嘘。这也再一次让中国药企看到了在创新药的产业链上还有多少路要走,也引发了对于踩中风口后如何把握机会的思考。-01-隔夜的金,还是到手的铜普米斯创始人刘晓林,曾是信达生物研发副总裁,在领导信迪利单抗的开发上市之后功成身退,于2018年自立门户创办了普米斯。普米斯的管线以靶向PD-L1、TIGIT、41BB的单抗、双抗为主。原本进展最快的是PD-L1/TGF-β双抗双抗,即PM8001,在同靶点赛道中属于第一梯队。但GSK和默克共同研发的PD-L1/TGF-β双抗双抗在2021年接连4项临床试验宣告失败,让其它追随者都默默放弃。在此之后,PM8002成为普米斯最大的指望,是全球首个且目前唯一进入Ⅲ期临床的PD-L1/VEGF双抗,在PD-(L)1/VEGF双抗的领域进度也属于前三,临床数据优异。除此之外,在研还有多款肿瘤和自免双抗、ADC、新型细胞疗法,已经陆续产生BD交易。刘晓林在创业初期融资比较顺利,2018年-2021年保持每年一轮融资的节奏,公司估值已经水涨船高。但普米斯的投资人不具备托举创新药上市的后期基金人脉,加上当时已经进入资本寒冬,在2021年之后再没有融资。普米斯的境况不算太糟,2022年起开始发力BD,将EGFR/cMet双特异抗体授权给翰森,首付款5000万元。次年这笔交易扩展到用于开发ADC,商业及里程碑付款提高到最高50亿元。2023年11月,普米斯与BioNTech达成授权协议,以5500万美元首付款和超十亿美元里程碑付款获得PD-L1/VEGF双抗的中国大区以外权益。这两笔BD交易,让普米斯到被收购前夕都拥有一定的现金流。因此,在PM8002卖给BioNtech时,公司并不是像很多其它biotech一样是走投无路下的无奈之举。与BioNTech的首次授权合作还是比较常规的,但引起话题的是后续的收购事件。2024年11月13日,普米斯与BioNTech达成股权收购协议。根据协议,BioNTech将以8亿美元预付款,最高1.5亿美元的里程碑付款的价码全额收购普米斯。这一收购价码甚至少于PD-L1/VEGF双抗单个资产的总交易额(详见:普米斯被BioNTech收购,一个“蛋比鸡贵”的时代),当时业内就在探讨为什么?一位从业者称,普米斯曾接触美国投资人,寻找上市机会。但可能是担心无法上市,在这之后不到6个月,普米斯就在现金流还不错的情况下,匆匆卖身。普米斯的考虑不无道理,两个进展最快的管线,一个暂停,一个授权出海,剩下的资产支持不了太高的估值。而如果不能上市,普米斯的现金流危机也早晚会出现。于是在熬着等机会,和早点上岸两个选项之间,普米斯选择了后者。“后轮投资人其实是反对的,但是早轮投资人主张卖身,想要尽快退出,而且没有咨询专业banker,所以没有谈下更高的价格。”与之形成对照的礼新,就找了经验丰富的banker谈判,在普米斯被9.5亿美金收购的同月,礼新研发的刚进I期的PD-1/VEGF双抗收到了近6亿美金首付。如果再坚持半年,普米斯可能能迎来长夜后的曙光,但历史的发展,往往没有如果。-02-普米斯的结局能避免吗?同一个管线,普米斯卖给BioNTech,潜在总金额十多亿美元;BioNTech卖给BMS,潜在总金额超过百亿美元。这其中有这个赛道大火带来的被动增值。在2024年世界肺癌大会上,康方生物发布临床研究数据显示,AK112在头对头3期临床试验中击败K药,一举抬高了PD-(L)1/VEGF双抗的价值。但更多的,BioNTech更是功不可没。普米斯的PD-L1/VEGF双抗授权给BioNTech后,已从二期迈入全球三期临床阶段。目前,BNT327联合化疗的二线三期试验已在华启动,全球性临床则聚焦一线治疗,目前已治疗了超过1,000名患者,包括针对广泛期小细胞肺癌和非小细胞肺癌的一线治疗的全球性3期试验,计划于2025年底前启动针对三阴性乳腺癌的全球性3期试验。BioNTech还计划通过超439例的三期研究全面验证疗效,同时加速探索BNT327与TROP2、HER2等靶点ADC药物的协同效应。如此规模的全球性临床是普米斯自己无法做到的,也是现在PM8002的价值所在。所以即使BioNTech获利更多,某国际知名美元基金合伙人依然认为,这就是个销售链条,没有BioNTech做“加工商”,卖不出这么多钱。“去年恒瑞引领的NewCo成为潮流,NewCo交易占据创新药交易事件的一半以上,也是一样的逻辑。需要加工商,把优质资产从成堆管线中筛选出来,带领中国创新药从国内走向国外,让别人看见并认可。被看见和被相信的成本是很高的。”三生制药没有经过中间商,一步到位和辉瑞达成授权合作,获得了12.5亿美元首付款和60亿美元潜在总付款。相比其他先授权给海外biotech,再寻找MNC买家的曲折路线,是一个非常成功的BD案例。但三生制药付出的时间成本是小biotech所难以承受的。很多时候,biotech只能见好就收,没有太多筹码。或许也是这一想法,促使普米斯早在PD-(L)1/VEGF双抗身价暴涨之前授权给BioNTech,此后更加被动。普米斯也不会想到,2024年,MNC和海外基金来华扫货,多款ADC和双抗/TCE出海,已经势不可挡,领头羊康方更是做出了惊艳的数据,紧跟其后的管线都可以借风口谈个好价格,比如礼新。如果不考虑时机,普米斯有没有争取更多价值的可能?答案也是肯定的。一位业内人士表示,避免贱卖优秀资产有几个要素,首先投资人要懂创新药研发和BD逻辑,最好有一定supportive network;其次biotech的BD要懂药物的未来市场、竞争格局和买方,且真心为公司着想;最后拥有处于临床阶段的爆款药物,和买方达成初步意向后,需要找banker介入配合。总的来说,还是人的问题。在一个理想的场景中:投资人一定要有长期主义,能在投资后长期监督和扶持;研发型创业者也需要为之计深远,找到专业和长期合作的基金,不要过于在意短期利益。有的时候,投资人为了抢项目给出过高的估值,之后可能反而成为公司下一步发展的绊脚石。对普米斯来说,核心管线进入更大的平台,投资人盈利退出,已经是一个皆大欢喜的结局,完成了从0-10的成功。而从10-100的巨大空间,留给其他中国biotech去思考。普米斯的案例,能够让中国创新药从业者看到优秀资产的价值天花板,在交易时做更充分的准备和更多的信心。......欢迎添加作者交流:吴妮:nora4409
2025-06-05
未上市药物面临的风险,就是股价总会随着各种临床数据起起落落文 | 凌馨编 | 王小图/视觉中国一位港股医药投研人士对《财经》说,当下的医药股,“涨跌全靠临床数据。”2025年前五个月,恒生创新药指数上涨35%,领涨恒生行业指数,涨幅是A股创新药指数的近3倍。其中,在恒生创新药成分涨幅排名前十的个股中,过半已经或被认为可能达成药物出海授权。6月4日,港股创新药领涨的是信达生物,涨幅达14.14%。原因是信达生物在研抗肿瘤药IBI363的临床数据公布了,被认为数据超预期,在肺癌治疗领域潜力巨大。与信达生物情况类似的还有再鼎医药、科伦博泰、复宏汉霖、荣昌生物、亚盛医药等。它们都在5月30日—6月3日举行的美国临床肿瘤学会(ASCO)期间,公开了重要药物试验数据,股价均有不同程度上涨。有涨的就有跌的。被视为中国创新药出海“领头羊”的康方生物,6月2日跌10.5%,其明星抗癌药依沃西的一项临床数据公布后,被认为未能达到预期。药企临床数据之所以如此受关注,背后是资本对这些药物的“出海”预期,这也是带动中国香港医药股2025年行情的一股重要力量。No.1临床数据背后的“出海”预期在2025年ASCO期间,中国药企至少有73项临床研究成果入选口头发言,其中仅达生物就有8项,带动股价大涨的是IBI363。IBI363是一款PD-1/IL-2α双抗,可用于肺癌、线结直肠癌、黑色素瘤等肿瘤治疗。信达生物公开IBI363的一系列数据后,摩根大通认为,该药与另一款信达生物研发的抗体偶联物(ADC)拥有较大潜力,预期信达可能将该两项资产授权出海或自行启动全球开发,于是新增两款候选药物的潜在美国市场销售预测。信达生物的IBI363研发目标,就是指向全球市场。公司创始人俞德超曾在2024年度业绩沟通会上介绍,该药用于治疗鳞状非小细胞肺癌和黑色素瘤的两个适应症,拿到了美国食品和药品监督管理局(FDA)的快速审评资格。康方生物5月30日披露的依沃西首个海外III期临床试验结果实验数据,之所以带来股价下跌,也与这款药品要赴美上市审批有关。这个试验结论是,“达到无进展生存期(PFS)的主要终点,且患者总生存期(OS)具有明显获益趋势。”依沃西在中国获批,靠的是PFS数据胜出帕博利珠单抗(俗称K药)。问题是,上述III期临床的OS数据为0.79,即依沃西+化疗,相比安慰剂+化疗,死亡风险降低21%,未达统计学显著差异。美国FDA审批肿瘤药物的“金标准”是OS,它才是直接反映患者生存获益的数据。四位港股投资者对《财经》表示,依沃西上述数据令人失望。毕竟,康方生物一度被海外投资者赋予中国创新药“DeepSeek”的意义,也是2025年这波港股创新药上涨中的翘楚,区间涨幅一度超过100%。不过,前述康方生物相关人士称,依沃西在海外的首个临床III期数据,“人种直接疗效和安全性无差异,国内数据在国际研究中很好地重现”。国内数据在海外可复现,至少印证了康方生物在国内做的临床试验,是可信的。并且,这个临床III期实验指向的是一个患者相对较少的小适应症,其最受关注的临床研究是针对一线肺癌治疗与K药的头对头试验,这才是真正指向500亿美元市场的大适应症。中国药企的出海BD(商务拓展),一大部分都是在相关药物海外临床得出好数据之后达成的。“其实都相信依沃西是好药,只是FDA一天没批、药物也没有真正形成销售,股价就总会随着各种临床数据起起落落。”前述港股医药投研人士告诉《财经》。这就是未上市药物面临的风险,也是一批“出海概念股”必将面对的未来。No.2从“卖孩子”到“抱大腿”?创新药出海,是支撑港股创新药2025年这波上涨的重要力量。“因为不少国内头部药企与跨国药企达成多项授权合作,印证了中国创新药领域在全球的竞争力。”一位公募基金医药基金经理表示。中国药企的出海BD,几乎都是在相关药物临床得出好数据之后达成的。2025年,港股出现了一批公开BD信息后大涨的创新药股,包括5月公布将收到辉瑞近百亿元首付款的三生制药,其股价三天涨了63.66%,还有与葛兰素史克(GSK)、百济神州等中外药企达成交易的映恩生物,将一款代谢病药物授权给默沙东的恒瑞医药等。从2025年初到5月30日,恒生创新药指数已经涨了45%;恒生指数年内涨幅最高的八大主题,均为医药题材,且涨幅均在35%以上,比排名第九的汽车主题高出至少10个百分点。同一时间,A股中证创新药指数涨幅13.47%,不及恒生创新药指数三分之一。在2025年前五个月,恒生创新药指数成分股中,涨幅最大个股中,三生制药、科伦博泰生物、诺诚健华、先声药业等排名前五的,均有出海授权交易达成。其中,三生制药涨幅超200%。“天下韭菜都一样,股价上去了你只会觉得自己没看懂。”前述医药投研人士承认,在2024年,自己对出海授权的理解还是“卖孩子”,担心中国创新药企将核心管线海外商业化权益授出,后续业绩增长乏力。然而,在2025年,授权跨国药企的行为,普遍被解读为“抱大腿”,这位医药投研人士的想法也在改变。6月2日,德国生物企业拜恩泰科(BioNTech)涨18.05%,靠的就是从中国买去的“孩子”——普米斯研发的PD-1/VEGF双抗。这是一款与依沃西类似靶点的抗癌药,拜恩泰科以最高可达111亿美元里程碑付款的价格,把普米斯研发的PD-1/VEGF双抗授权给了百时美施贵宝(BMS)。拜恩泰科在2023年买下了PD-1/VEGF双抗相关授权,并于2024年全资收购了普米斯,彼时花费也就十多亿美元。一转手,仅预付款和年度无条件付款,拜恩泰科拿到了35亿美元,未来甚至可达90多亿美元。普米斯可能就此错失了至少20亿美元的净收入,以及未来的更多可能性。但积极看,一个融资额总计2.4亿美元的B轮公司,如果不靠跨国药企和中间商,也很难在未来市场中占据大份额。“跨国药企和海外企业的BD交易,有‘背书’的作用。”一位一级市场医药投资人士告诉《财经》,这是她判断药物潜力和企业研发实力的重要判断标准,“因为我可能不专业,可能对一些细分领域没有那么懂,但跨国药企的研发、尽调,肯定是最专业的”。不仅是未盈利创新药企,中国大型传统药企也纷纷通过BD实现“出海”。6月2日,翰森制药与美国再生元(Regeneron)共同宣布,再生元以8000万美元首付款、最高19.3亿美元里程碑付款获得翰森在研GLP-1/GIP双受体激动剂海外授权。从市场目前的表现看,“出海潜力”仍然是左右投资机构对创新药看法一个重要指标。往期 · 推荐别错过优质内容,记得设置星标或在看哟
100 项与 翰森製藥集團有限公司 相关的药物交易
登录后查看更多信息
100 项与 翰森製藥集團有限公司 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
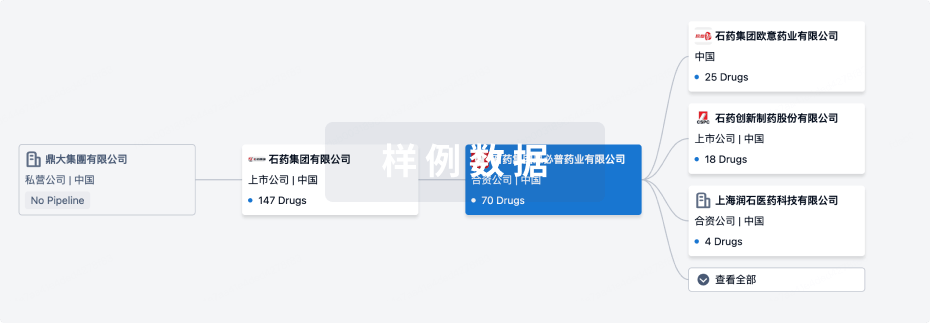
管线布局
2025年08月20日管线快照
无数据报导
登录后保持更新
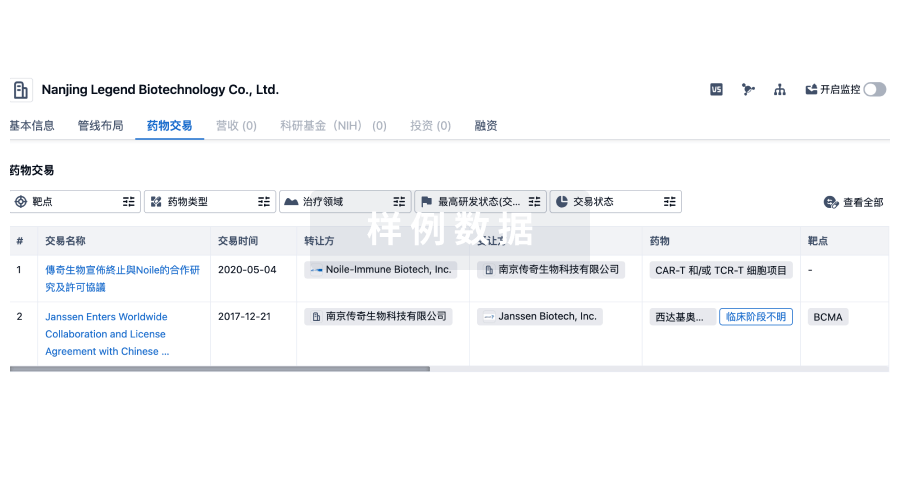
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
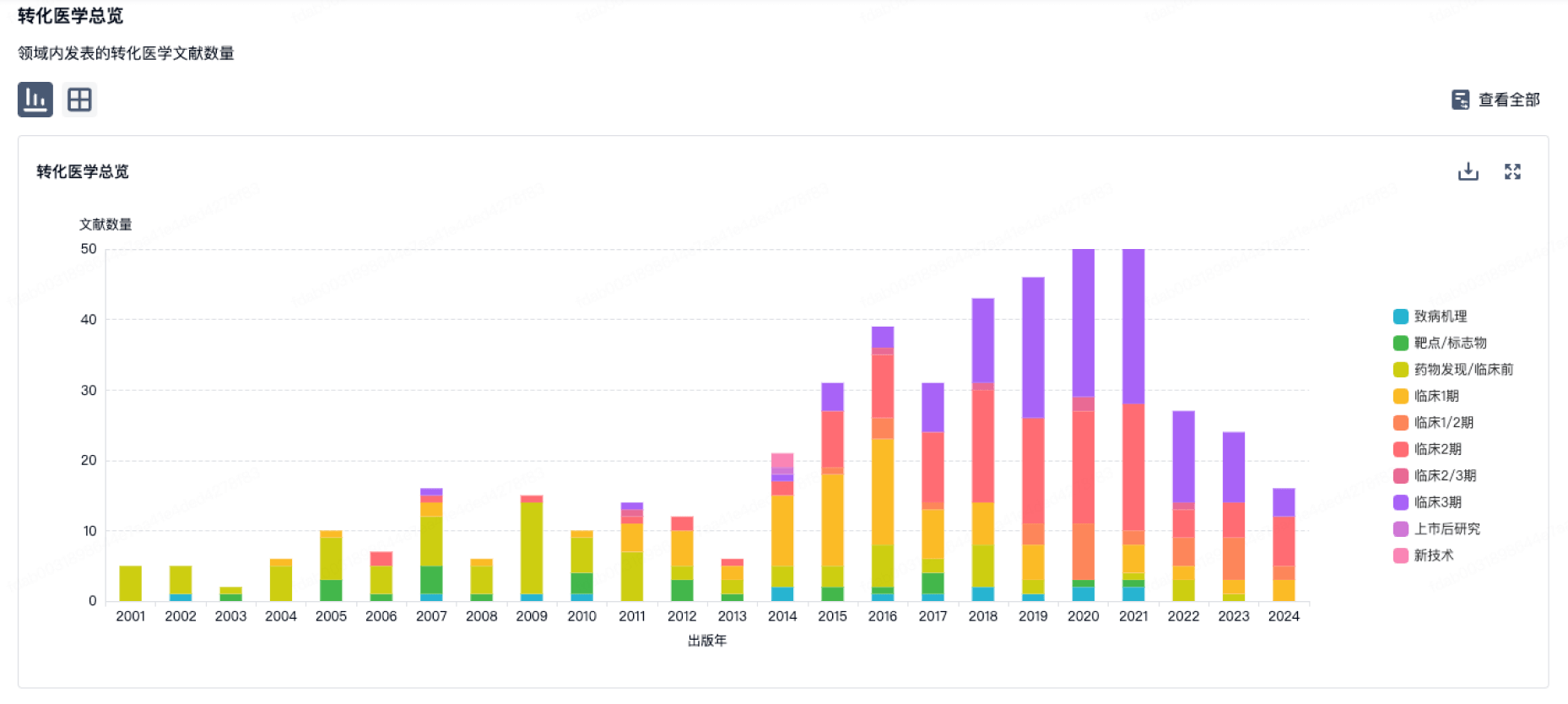
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
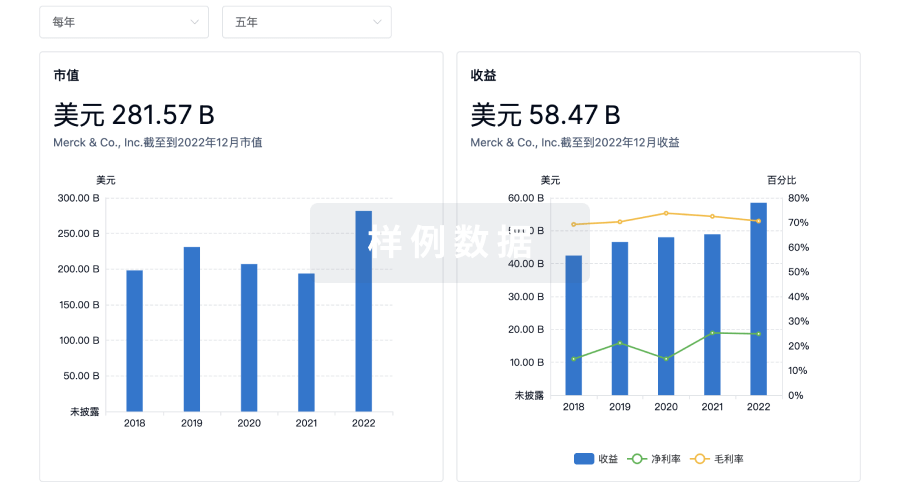
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或
投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或
融资
发掘融资趋势以验证和推进您的投资机会。
登录
或
Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用