预约演示
更新于:2025-03-08

Cryofocus Medtech (Shanghai) Co. Ltd.
更新于:2025-03-08
概览
关联
1
项与 康沣生物科技(上海)股份有限公司 相关的临床试验NCT06378671
Single-center Clinical Study on the Safety and Effect of Bronchoscopic Cryotherapy in Patients With Chronic Cough
Chronic cough is one of the most common complaints in respiratory specialty clinics, imposing significant economic burden on patients and severely affecting their quality of life. Currently, the pathogenesis of chronic refractory cough remains incompletely understood, and treatment remains a major challenge in clinical practice. Cryotherapy treatment via bronchoscopy has shown efficacy in certain airway diseases, but there is currently no research reporting its effects on chronic refractory cough.
The goal of this clinical trial is to learn if Cryotherapy treatment works to treat individuals with chronic cough. It will also learn about The safety and effectiveness of the cryotherapy treatment system produced by Ningbo SensCure Biotechnology Co., Ltd.. The main questions it aims to answer are:
Does cryotherapy treatment lower the frequency and severity of cough and enhance quality of life?
Will there be safety or operational performance issues when using this cryotherapy treatment system? Researchers will compare cryotherapy treatment with no treatment to determine if cryotherapy treatment is effective for treating chronic cough.
Participants will:
Take routine bronchoscopy examination, lavage, and mucosal biopsy ,with/without cryotherapy treatment locally (around the left and right main bronchi, upper trachea, and carina)
undergo a screening period of approximately 28 days. Follow-up visits and necessary examinations will be scheduled for the 3rd day after treatment initiation and at weeks 1, 2, 4, 8, and 12 thereafter.
Monitor vital signs and clinical manifestations.
The goal of this clinical trial is to learn if Cryotherapy treatment works to treat individuals with chronic cough. It will also learn about The safety and effectiveness of the cryotherapy treatment system produced by Ningbo SensCure Biotechnology Co., Ltd.. The main questions it aims to answer are:
Does cryotherapy treatment lower the frequency and severity of cough and enhance quality of life?
Will there be safety or operational performance issues when using this cryotherapy treatment system? Researchers will compare cryotherapy treatment with no treatment to determine if cryotherapy treatment is effective for treating chronic cough.
Participants will:
Take routine bronchoscopy examination, lavage, and mucosal biopsy ,with/without cryotherapy treatment locally (around the left and right main bronchi, upper trachea, and carina)
undergo a screening period of approximately 28 days. Follow-up visits and necessary examinations will be scheduled for the 3rd day after treatment initiation and at weeks 1, 2, 4, 8, and 12 thereafter.
Monitor vital signs and clinical manifestations.
开始日期2024-04-01 |
申办/合作机构  广州医科大学 广州医科大学 [+1] |
100 项与 康沣生物科技(上海)股份有限公司 相关的临床结果
登录后查看更多信息
0 项与 康沣生物科技(上海)股份有限公司 相关的专利(医药)
登录后查看更多信息
2
项与 康沣生物科技(上海)股份有限公司 相关的文献(医药)2019-12-01·Trials4区 · 医学
Efficiency and safety of renal denervation via cryoablation (Cryo-RDN) in Chinese patients with uncontrolled hypertension: study protocol for a randomized controlled trial
4区 · 医学
ArticleOA
作者: Ji, Meng ; Shen, Li ; Chen, Han ; Qiao, Lingjuan ; Xu, Yawei ; Ge, Junbo ; Zhang, Yi
Abstract:
Background:
Clinical data show that due to the limited effects of lifestyle regulation and unsatisfactory drug adherence, only half of the hypertensive population have their blood pressure (BP) under control. In recent years, catheter-based renal denervation (RDN) has been used as a novel approach for treating uncontrolled hypertension. The safety and efficacy of catheter-based RDN have been confirmed by a number of studies and trials in which the participants were all non-Chinese and RDN was conducted via radiofrequency or ultrasound.
Methods/design:
This study is a prospective multicenter randomized sham-controlled trial that aims to investigate the safety and efficacy of cryoablation RDN (cryo-RDN) using a novel dedicated cryoablation balloon catheter (Cryofocus, China). A total of 200 Chinese patients who have uncontrolled hypertension despite standard medical treatment will be enrolled. With drug standardization, eligible participants will be randomized in a 1:1 ratio to undergo cryo-RDN treatment or renal angiography alone as a sham treatment.The primary endpoint is defined as the change in 24-h ambulatory systolic blood pressure from baseline to 6 months. Office BP and other 24-h ambulatory BP are included as secondary endpoints. Safety endpoints primarily include any adverse effects.
Discussion:
This study was designed to verify the safety and efficacy of cryo-RDN with Cryofocus balloon catheters in uncontrolled hypertensive patients on polypharmacy. The aim is to provide a new way to improve the control of hypertension in China as a complement to drug therapy.
Trial registration:
ChiCTR, ChiCTR1800017707. Registered on 10 August 2018.
2019-12-01·Trials
Correction to: Efficiency and safety of renal denervation via cryoablation (Cryo-RDN) in Chinese patients with uncontrolled hypertension: study protocol for a randomized controlled trial
作者: Ji, Meng ; Shen, Li ; Chen, Han ; Qiao, Lingjuan ; Xu, Yawei ; Ge, Junbo ; Zhang, Yi
After publication of our article [1] we were notified that the word “References” was wrongly included in the title.
1
项与 康沣生物科技(上海)股份有限公司 相关的新闻(医药)2022-11-29
DUBLIN--(
BUSINESS WIRE
)--The
"Surgical Devices Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update"
report has been added to
ResearchAndMarkets.com's
offering.
This report provides comprehensive information about the Surgical Devices pipeline products with comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
Report Scope
Extensive coverage of the Surgical Devices under development
The report reviews details of major pipeline products which includes, product description, licensing and collaboration details and other developmental activities
The report reviews the major players involved in the development of Surgical Devices and list all their pipeline projects
The coverage of pipeline products based on various stages of development ranging from Early Development to Approved/Issued stage
The report provides key clinical trial data of ongoing trials specific to pipeline products
Recent developments in the segment/industry
The report enables you to:
Formulate significant competitor information, analysis, and insights to improve R&D strategies
Identify emerging players with potentially strong product portfolio and create effective counter strategies to gain competitive advantage
Identify and understand important and diverse types of Surgical Devices under development
Develop market-entry and market expansion strategies
Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
In-depth analysis of the product's current stage of development, territory and estimated launch date
Key Topics Covered
1 Table of Contents
1.1 List of Tables
1.2 List of Figures
2 Introduction
2.1 Surgical Devices Overview
3 Products under Development
3.1 Surgical Devices - Pipeline Products by Stage of Development
3.2 Surgical Devices - Pipeline Products by Segment
3.3 Surgical Devices - Pipeline Products by Territory
3.4 Surgical Devices - Pipeline Products by Regulatory Path
3.5 Surgical Devices - Pipeline Products by Estimated Approval Date
3.6 Surgical Devices - Ongoing Clinical Trials
4 Surgical Devices - Pipeline Products under Development by Companies
4.1 Surgical Devices Companies - Pipeline Products by Stage of Development
4.2 Surgical Devices - Pipeline Products by Stage of Development
5 Surgical Devices Companies and Product Overview
6 Surgical Devices - Recent Developments
7 Appendix
7.1 Methodology
List of Tables
Surgical Devices - Pipeline Products by Stage of Development
Surgical Devices - Pipeline Products by Segment
Surgical Devices - Pipeline Products by Territory
Surgical Devices - Pipeline Products by Regulatory Path
Surgical Devices - Pipeline Products by Estimated Approval Date
Surgical Devices - Ongoing Clinical Trials
Surgical Devices Companies - Pipeline Products by Stage of Development
Surgical Devices - Pipeline Products by Stage of Development
Glossary
List of Figures
Surgical Devices - Pipeline Products by Stage of Development
Surgical Devices - Pipeline Products by Segment
Surgical Devices - Pipeline Products by Territory
Surgical Devices - Pipeline Products by Regulatory Path
Surgical Devices - Pipeline Products by Estimated Approval Date
Surgical Devices - Ongoing Clinical Trials
Companies Mentioned
6S Medical LLC
Abbott Operations Uruguay Srl
Acoustic MedSystems Inc
Aesculap Inc
Anastom Surgical
Anchora Medical Ltd
Apercure Surgical Ltd
Apollo Endosurgery Inc
Applied Medical Resources Corporation
Apyx Medical Corp
Archon Medical Technologies LLC
Augusta University
Baxter International Inc
BioTex Inc
BriteSeed
BTG International Inc
Carnegie Mellon University
Centre Hospitalier Universitaire de Nice
Charite University Hospital of Berlin
Chengdu Yurui Innovation Technology Co Ltd
Colospan Ltd
Control Medical Technology LLC
Core Essence Orthopaedics, LLC
Corporis Medical
Covidien Ltd
CR Bard Inc
Creative Balloons GmbH
Cryofocus Medtech Shanghai Co Ltd
CT Resources Inc
Cutera Inc
Cypris Medical, Inc.
Dartmouth College
Deep Blue Medical Advances Inc
Drexel University
Emblation Ltd
Embricon Ltd (Inactive)
Endo Tools Therapeutics SA
EndoEvolution, LLC
Ethicon Endo-Surgery Inc
ExpandoHeat LLC
Fixit Medical Ltd
Fogless International AB
G.I. Windows Inc
Galil Medical Ltd
Garantis
Genicon Inc
GI Windows Medical Corp
Grand Valley State University
H. Lee Moffitt Cancer Center & Research Institute Inc
Hangzhou Kangsheng Medical Equipment Co Ltd
Hannover Medical School
Harvard University
Hospital for Special Surgery
IceCure Medical Ltd
Imperial College London
Indian Institute of Technology Delhi
Innovex Medical Co Ltd
Integra LifeSciences Holdings Corp
Intuitive Surgical Inc
IonMed Ltd.
Jinan Micro Intelligent Technology Co Ltd
Johns Hopkins University
Keren Medical Ltd.
LeMaitre Vascular Inc
Livsmed Inc
LuSeed Vascular
Lymphatica Medtech SA
MagniFeel Surgical Instruments
Mayo Clinic
Mecmaan Healthcare Ltd
Medical University of South Carolina
MediShield B.V.
Medtronic Plc
Mel Frontier Co Ltd
Meta Biomed Co Ltd
Michigan State University
Naval Medical Research Center
Next Science Ltd
NorthShore University Health System
NOvate Medical Technologies, LLC
NoviRad Inc
NovoGI LTD
Novolock Ltd
Novuson Surgical, Inc.
Ohio State University
Oklahoma State University
Olympus America Inc
OV World Co Ltd
Pacira BioSciences Inc
Parker Hannifin Corp
Pavmed Inc
PetVivo Holdings Inc
Physcient, Inc.
Polytechnic University of Catalonia
Pro-Dex Inc
Queen Mary University of London
Ra Medical Systems Inc
Ramona Optics Inc
Ratner BioMedical Inc
Resultados y Calidad del Sistema Sanitario Publico de Andalucia
Rice University
Saint Louis University
Samyang Biopharmaceuticals Corp
Sanovas Inc
Shanghai MicroPort Medical Group Co Ltd
Shanghai Shengzhe Medical Technology Co Ltd
Shanghai Yichao Medical Equipment Co Ltd
Silmag SA
simedeq
Simon Fraser University
StatLink Surgical
STRATA Skin Sciences Inc
Stryker Corp
SurgyAid, LLC
Sutura Inc
Teleflex Inc
Tepha Inc
The Feinstein Institute for Medical Research
TransMed7 LLC
Trinity College Dublin
University of Auckland
University of California
University of California Davis
University of California Los Angeles
University of California San Diego
University of Central Florida
University of Chicago
University of Maryland
University of Maryland Baltimore
University of Minnesota
University of Missouri
University of North Carolina
University of South Florida
University of Strasbourg
University of Texas at Austin
University of Texas Medical Branch at Galveston
University of Toledo
University of Utah
University of Virginia
US Medical Innovations LLC
USGI Medical Inc
Vanderbilt University
Vascular Insights LLC
VB Devices
Vessi Medical Ltd
Viveve Medical Inc
Viveve, Inc.
Wake Forest Baptist Medical Center
Weill Cornell Medical College
West Virginia University
X-Biomedical Inc
For more information about this report visit
https://www.researchandmarkets.com/r/ekyrrp
Source: GlobalData
100 项与 康沣生物科技(上海)股份有限公司 相关的药物交易
登录后查看更多信息
100 项与 康沣生物科技(上海)股份有限公司 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
管线布局
2025年04月26日管线快照
无数据报导
登录后保持更新
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
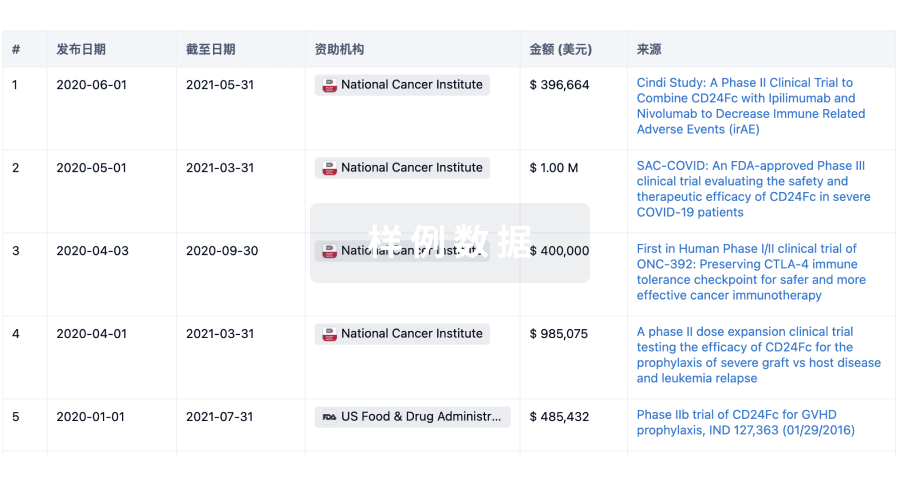
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或
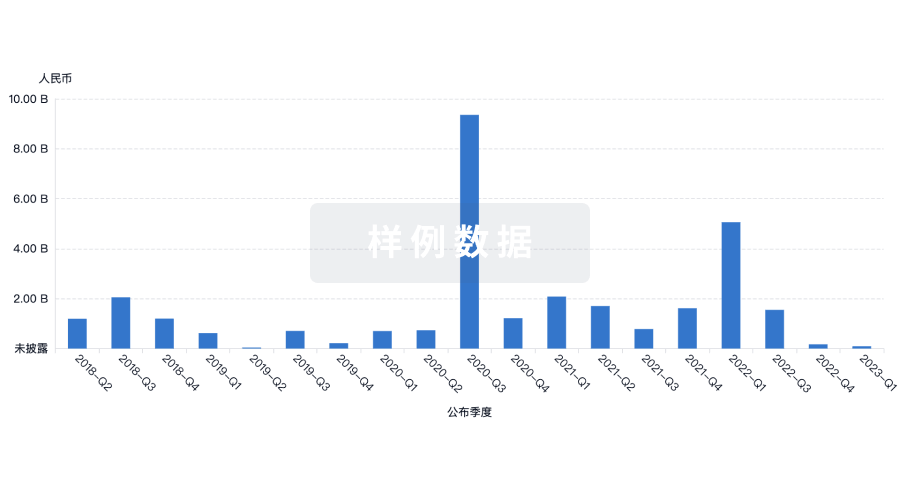
投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或
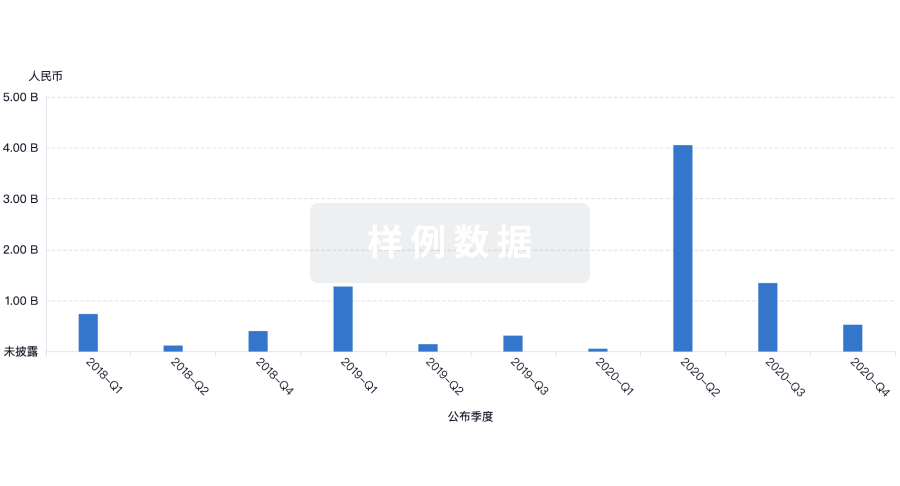
融资
发掘融资趋势以验证和推进您的投资机会。
登录
或
来和Eureka LS聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用