更新于:2024-11-01

Florida Urology Partners LLP
更新于:2024-11-01
概览
关联
100 项与 Florida Urology Partners LLP 相关的临床结果
登录后查看更多信息
0 项与 Florida Urology Partners LLP 相关的专利(医药)
登录后查看更多信息
6
项与 Florida Urology Partners LLP 相关的新闻(医药)2024-07-23
Clinical study data to evaluate the safety and efficacy of the Neuspera System for the treatment of urinary urge incontinence (UUI) will support the FDA submission for regulatory approval
SAN JOSE, Calif., July 23, 2024 /PRNewswire/ -- Neuspera Medical, Inc., a neuromodulation company pioneering the Neuspera Implantable Sacral Neuromodulation (SNM) System, announced it has reached a significant milestone: All implanted patients have completed the 6-month primary endpoint visit in the SANS-UUI pivotal trial of the Neuspera System. The Neuspera System is a discreet, minimally invasive, ultra-miniaturized implant designed to provide patients personal control and relief from urinary urge incontinence (UUI), a symptom of overactive bladder (OAB). The clinical data will support the U.S. Food and Drug Administration (FDA) submission for regulatory approval of the Neuspera System to treat OAB symptoms.
"We're grateful to every physician and patient who participated in the clinical trial, and we're excited that our uniquely differentiated technology will help millions of patients when it is FDA-approved for treatment of OAB symptoms," said Steffen Hovard, CEO of Neuspera Medical. "The fact that the market is only 5% penetrated at this time is a clear indication that patients are looking for something different than what's available today. The Neuspera System delivers true innovation."
OAB is a common medical disorder affecting roughly one in six adults. OAB symptoms include a sudden, uncontrolled need or urge to urinate, urine leakage, and/or the need to pass urine many times during the night and day. These symptoms can make it difficult to work, socialize, exercise and lead a normal, active life which can lead to depression, self-esteem and social stigma issues.
This milestone comes on the heels of the American Urological Association (AUA) and Society for Urodynamics, Female Pelvic Medicine and Urogenital Reconstruction's (SUFU) recently published 2024 Guideline on the Diagnosis and Treatment of Idiopathic Overactive Bladder. The guideline promotes a shared decision-making process between the patient and physician that weighs the patient's comorbidities along with expressed values, preferences, and treatment goals in order to help them make an informed decision regarding treatment. This new guideline will facilitate OAB patient access to the therapy that best meets their particular needs and goals without requiring a lengthy stepwise approach through multiple levels of therapy. SNM is recognized as the "gold standard" neuromodulation therapy for OAB. With a dramatically smaller implant and incision size than anything else on the market, and efficacy comparable to the best SNM data[1], Neuspera is expected to benefit greatly from the new guideline's consultative approach providing accelerated patient access to minimally invasive therapies that were previously considered "third line."
The SANS-UUI multi-center, single-arm clinical study was designed to demonstrate the safety and efficacy of the Neuspera System and gain FDA approval in the U.S. Patients were screened and implanted with the device at 26 centers across the U.S. and Europe. Patients who participated in the Phase One clinical trial – and who have been finding relief using the Neuspera System to successfully control their UUI symptoms for up to 3.5 years – have called the Neuspera System "life-changing," "the miracle," and "easy to do."
"I love that there is no battery implant compared to traditional devices and that it's outside of my body and I'm in charge of it," said one patient who participated in the Phase One clinical trial.
"It's improved my life so much that I tell everybody about it," another patient said.
"Reaching the final patient's primary endpoint visit for the clinical trial is an important milestone in Neuspera's journey towards revolutionizing the way physicians utilize SNM therapy," said Dr. Steve Siegel, chief medical officer of Neuspera Medical. "This will be a great solution for patients who want a high degree of symptom control with the smallest implantable neurostimulator available, designed to empower patients and provide ultimate discreetness putting control of life-altering symptoms in patients' hands."
"As the coordinating investigator for the SANS-UUI clinical trial, based on my experience with the Neuspera System and how patients are responding, I'm excited to soon be able to offer an OAB treatment option that truly gives patients control of their bladders in the least invasive way with the ability to self-administer their therapy when it's convenient for them, and nobody else can see or feel it," said Dr. Osvaldo Padron with Florida Urology Partners.
The Neuspera System has FDA clearance for treating chronic pain of peripheral nerve origin and is currently an investigational device for treating UUI, a symptom associated with OAB.
About Neuspera Medical
Neuspera Medical, Inc. is committed to developing implantable medical devices that will improve the lives of patients battling chronic illnesses. The Neuspera platform is designed to provide patients and physicians with new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives aim to help patients restore their health and well-being for a better quality of life. For more information, please visit .
The Neuspera System is the least invasive sacral neuromodulation device and includes an ultra-miniaturized pulse generator attached to an electrode array. It is designed to be discreet and fit within the protected space of the sacral foramen, so patients typically don't feel any lump or bulge and the tiny 4-5 mm scar from the implant procedure is usually barely perceptible, even if you know where to look. This design eliminates the need for more invasive tunneling and a separate pocket for an implanted battery, avoiding issues that negatively affect patient satisfaction with traditional SNM systems while empowering patients to regain control of their OAB symptoms and life.
1 Based on outcomes of the SANS-UUI Phase I clinical data; Padron et al. (2021). TREATMENT OF URINARY URGENCY INCONTINENCE (UUI) WITH AN ULTRA-MINIATURIZED SACRAL NERVE MODULATION (SNM) SYSTEM: PRELIMINARY OUTCOMES OF THE SANS-UUI STUDY. Journal of Urology. 2021 Sep 1;206(Supplement 3):e1158.
Media Contact:
Shelli Lissick
Bellmont Partners
651-276-6922
[email protected]
SOURCE Neuspera Medical, Inc.
临床1期
2024-02-28
Participants now being recruited for RESTORE study to expand the evidence base for Revi, a minimally invasive, FDA-authorized, patient-centric tibial neuromodulation therapy
PARK CITY, Utah, Feb. 28, 2024 /PRNewswire/ -- BlueWind Medical, Ltd. today announced that Dr. Osvaldo Padron of Florida Urology Partners in Tampa, Florida, has treated the first patient in the RESTORE study, a prospective, multi-center, open label, post market, randomized controlled trial. The RESTORE study will recruit 150 patients at up to 20 centers in the United States.
Revi is an innovative implantable Tibial NeuroModulation (iTNM) device that offers safe and effective treatment for UUI, as demonstrated in the OASIS pivotal study. Revi is placed near the ankle in a single predictable procedure under local anesthesia and is powered by a lightweight external wearable that is used at the patient's convenience once or twice daily to provide therapy. When activated, the Revi Implant stimulates the posterior tibial nerve to provide relief from UUI. Revi received FDA marketing authorization in August 2023, with no conditions of approval or post market study requirements. Revi is the only neuromodulation therapy on the market that allows physicians
to use their discretion to determine whether the patient should fail or not tolerate more conservative treatments before using the Revi System rather than mandating "step-therapy."
"Urge urinary incontinence is disruptive, embarrassing, and causes undue stress in people's lives. Current treatments work for some people, but not most. There has been a real need for a more patient-centric and minimally invasive treatment option for people," said Dr. Padron. "I am pleased to have treated the first patient with the Revi System as part of the RESTORE study, an important clinical study that will help advance treatment options for people who suffer from urge urinary incontinence. Our site is recruiting additional qualifying study participants, and I encourage people to apply."
"This is a great day in our collective journey to bring more convenient, comfortable, and patient-centric treatment options to people suffering from the debilitating symptoms of urge urinary incontinence," said Dan Lemaitre, Chief Executive Officer of BlueWind Medical. "We are so grateful to Dr. Padron for treating the first patient in this critical research effort to help transform the treatment paradigm for UUI."
About the RESTORE study:
RESTORE is a post market randomized control trial to compare the efficacy of the Revi™ System Therapy On versus Off in Reducing Urinary Urge Incontinence Episodes. BlueWind Medical elected to initiate this trial to build on the body of evidence supporting the efficacy of the Revi System in reducing urge urinary incontinence (UUI) symptoms. The study is currently recruiting participants. To determine if you may qualify, please visit BlueWind Medical's study webpage. You can also find more details about the RESTORE study (NCT06217328) on clinicaltrials.gov.
About BlueWind Medical Ltd.
BlueWind Medical is transforming the field of neuromodulation therapy through the development of innovative, patient-centric medical technology for the treatment of disease. BlueWind is committed to enhancing the quality of life and overall wellbeing of patients with an initial focus on those living with urgency urinary incontinence (UUI). BlueWind's Revi System is the first and only implantable tibial neuromodulation device activated by a battery-operated external wearable to receive FDA marketing authorization for patients with UUI.
For additional information about BlueWind Medical, please visit .
SOURCE BlueWind Medical
上市批准
2023-12-06
The international study is evaluating the safety and efficacy of the Neuspera System for the treatment of urinary urge incontinence (UUI), a symptom of overactive bladder (OAB)
SAN JOSE, Calif., Dec. 6, 2023 /PRNewswire/ -- Neuspera Medical, Inc., a neuromodulation company pioneering the Neuspera Implantable Sacral Neuromodulation (SNM) System, today announced it has completed patient enrollment and implant procedures for the pivotal clinical trial (SANS-UUI) of its Neuspera System, a discreet, minimally invasive implant designed to provide patients personal control and relief from urinary urge incontinence (UUI), a symptom of overactive bladder (OAB).
"We are pleased to have reached the target patient enrollment in our pivotal SANS-UUI trial," said Steffen Hovard, CEO of Neuspera Medical. "The completion of patient implants builds on the progress and strong momentum from our previously reported positive clinical outcomes."
The SANS-UUI multi-center, single-arm clinical study is designed to demonstrate the safety and efficacy of the Neuspera System and gain U.S. Food & Drug Administration (FDA) approval in the U.S. Patients were screened and implanted with the device at 26 centers across the U.S. and Europe.
"Completing patient enrollment and implants is an important milestone in Neuspera's journey towards revolutionizing the way physicians utilize SNM therapy," commented Dr. Steve Siegel, Chief Medical Officer of Neuspera Medical. "We're grateful to the patients and physicians partnering with us in this study, and we look forward to finalizing the data that will support our FDA submission of the Neuspera System."
OAB is a common medical disorder affecting roughly 1 in 6 adults. OAB symptoms include a sudden, uncontrolled need or urge to urinate, urine leakage, and/or the need to pass urine many times during the night and day. These symptoms can make it difficult to work, socialize, exercise and lead a normal, active life which can lead to depression, self-esteem and social stigma issues.
Neuspera's unique system is designed to provide patients who have failed medication management a minimally invasive, more approachable way of benefiting from sacral neuromodulation, a clinically proven effective treatment that's been used for more than 30 years to treat UUI associated with OAB.
The Neuspera System is the least invasive sacral neuromodulation device and includes an ultra-miniaturized pulse generator attached to an electrode array. It is designed to be discreet, to provide patients with freedom from inconvenient and visible batteries, and to empower them to regain control of their life.
"As the coordinating investigator for the SANS-UUI clinical trial, I'm excited at the prospects of the Neuspera System for treating patients with OAB symptoms in the least invasive manner," said Dr. Osvaldo Padron with Florida Urology Partners.
The Neuspera System has FDA clearance for treating chronic pain of peripheral nerve origin and is undergoing clinical trials as an investigational device for the use of treating UUI, a symptom associated with OAB.
About Neuspera Medical
Neuspera Medical, Inc. is committed to developing implantable medical devices that will improve the lives of patients battling chronic illnesses. The Neuspera platform is designed to provide patients and physicians with new, and potentially earlier, treatment options that are less invasive and more adaptable. These therapeutic alternatives aim to help patients restore their health and well-being for a better quality of life. For more information, please visit .
Media Contact:
Hyedi Nelson
Bellmont Partners
651-757-7054
[email protected]
SOURCE Neuspera Medical, Inc.
临床研究上市批准
100 项与 Florida Urology Partners LLP 相关的药物交易
登录后查看更多信息
100 项与 Florida Urology Partners LLP 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
管线布局
2024年11月10日管线快照
无数据报导
登录后保持更新
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

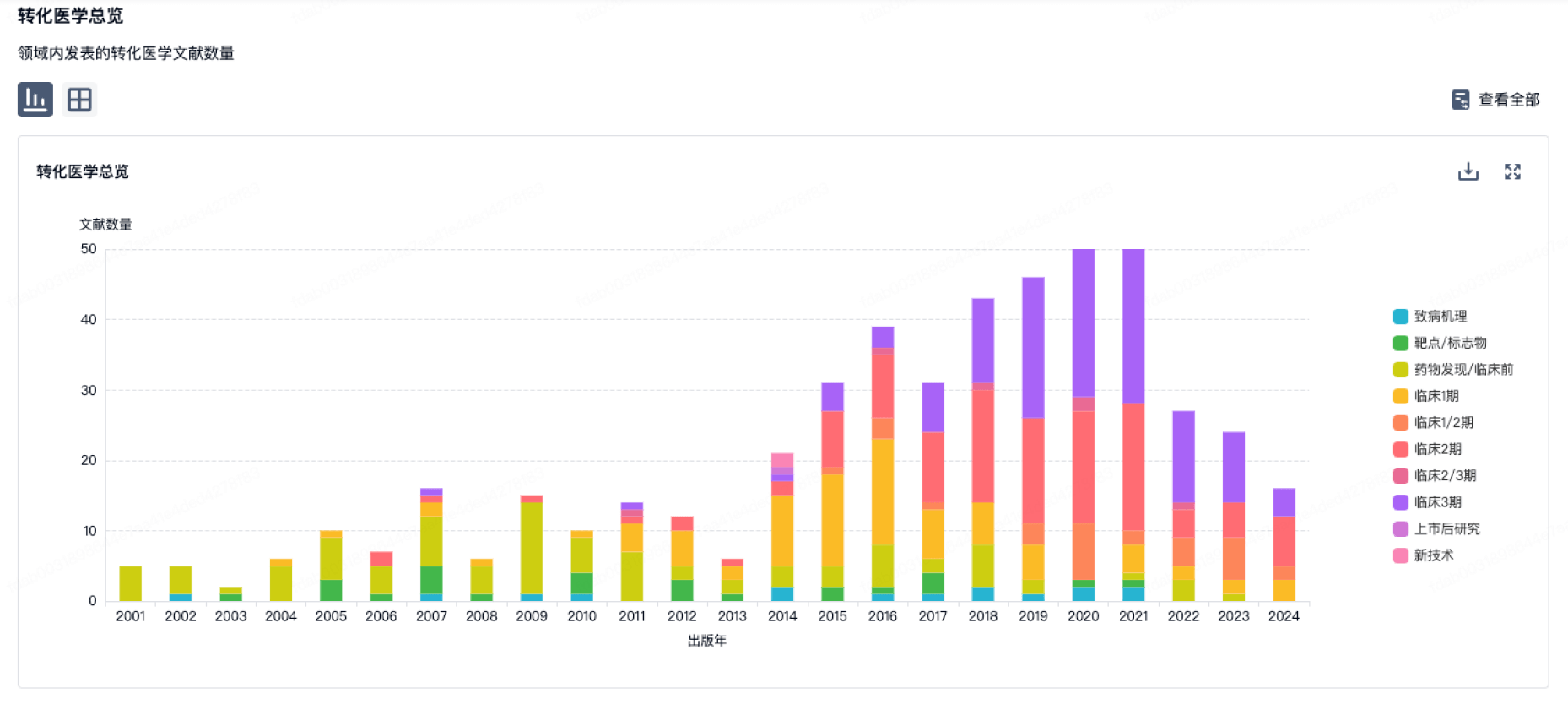
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用