更新于:2024-11-01

Beijing Genetron Biotechnology Co. Ltd.
更新于:2024-11-01
概览
关联
100 项与 北京泛生子生物科技有限公司 相关的临床结果
登录后查看更多信息
0 项与 北京泛生子生物科技有限公司 相关的专利(医药)
登录后查看更多信息
3
项与 北京泛生子生物科技有限公司 相关的文献(医药)2019-09-10·Blood Advances2区 · 医学
Persistence of skewed X-chromosome inactivation in pre-B acute lymphoblastic leukemia of a female ATRX mutation carrier
2区 · 医学
Article
作者: Battiwalla, Minoo ; Hauffe, Sara ; Foster, Matthew C. ; Montgomery, Nathan D. ; Oetjen, Karolyn A. ; Ito, Sawa ; Calvo, Katherine R. ; Bradley, Christian P. ; Chen, Cai ; Yuan, Constance ; Barrett, A. John ; Panjwani, Reema ; Gibbons, Richard J. ; Yan, Cheng ; Patel, Prapti Arvind
2019-07-01·Cancer Research
MTAP Loss Promotes Stemness in Glioblastoma and Confers Unique Susceptibility to Purine Starvation
Article
作者: Keir, Stephen T. ; Sun, Ran ; Spasojevic, Ivan ; Sampson, John H. ; Hansen, Landon J. ; Friedman, Henry S. ; Healy, Patrick ; Zhao, Fangping ; He, Yiping ; Chen, Chin-Pu J. ; Greer, Paula K. ; Carpenter, Austin B. ; Moure, Casey J. ; Yang, Rui ; Singh, Simranjit X. ; Chen, Lee H. ; Bigner, Darell D. ; Grenier, Carole ; Hemphill, Carlee ; Pirozzi, Christopher J. ; Murphy, Susan K. ; Huang, Zhiqing ; Ruger, Ryan C. ; Yan, Hai ; McLendon, Roger E. ; Herndon, James E. ; Friedman, Allan H.
2019-03-18·Neuro-Oncology1区 · 医学
Sensitive and rapid detection ofTERTpromoter andIDHmutations in diffuse gliomas
1区 · 医学
Article
作者: Bigner, Darell D ; Liu, Heng ; Diplas, Bill H ; Yang, Rui ; Hansen, Landon J ; Zachem, Alexis L ; Waitkus, Matthew S ; Zhao, Fangping ; Yan, Hai ; Jiao, Yuchen ; McLendon, Roger E ; He, Yiping
127
项与 北京泛生子生物科技有限公司 相关的新闻(医药)2024-10-04
·动脉网
“基因测序仪,怎么也开始走手机和新能源汽车的老路了。”当国产基因测序仪厂商纷纷举办隆重的发布会来为自家新品造势,有从业者如此感叹。而这样的场景,在国内以往的生命科学工具行业里,可谓见所未见。
基因测序仪一直被视为实验工具皇冠上的明珠,神秘而稀有。如今,基因测序仪的开发者们主动站在聚光灯下,讲述自己的开发细节和产品性能,和台下成百上千的使用者分享这个黑盒子的无限可能,或许人人拥有自己基因组的时代已经不远。
而在这个过程中,像雨后春笋一样冒出来的国产基因测序仪,无疑会产生举足轻重的价值。但基因测序仪的商业市场理性而残酷,国产基因测序仪能否在被海外品牌把持的市场夹缝中生根发芽?哪些测序仪能够长久留在舞台上?仍是未知数。
国产基因测序仪风起云涌
在造基因测序仪这件事上,国人的工匠精神被发挥到极致。
眼下,国产基因测序仪已经基本度过了从无到有的艰难探索阶段,进入品牌和机型都快速增长的新周期。2023年被视为国产基因测序仪的商业化元年,各大品牌纷纷发布成立以来的首款商业化机型。到了2024年,不少国产基因测序仪品牌已经开始形成产品矩阵。总体而言,现阶段的国产基因测序仪市场呈现出性能的极致性、功能的多样性、技术的全面性等特点。
首先来看性能。国产基因测序仪高举高打,无论是测序的通量、读长还是速度,都有产品做到了全球最高水平。基因测序的通量,是指单位时间内可以检测出的碱基序列数量,是衡量基因测序仪性能的重要指标。二代测序技术的出现,让基因测序快速在科研和临床场景中普及开,背后的主要原因就在于通量的急剧提升。通常,二代测序技术能够一次并行对几十万到几百万条DNA分子进行序列测定,同时产生数千条至数百万条测序片段,而此前主要采用的Sanger测序法,每次只能测序一小段DNA。
目前,已经有多家国内基因测序仪厂商推出了超高通量基因测序仪,单次运行可以产生Tb数量级别的碱基序列。比如,华大智造的DNBSEQ-T20×2、DNBSEQ-T10×4RS、DNBSEQ-T7,真迈生物的SURFSeq 5000、赛陆医疗的Salus EVO等,都属于超高通量基因测序仪,单次测序通量都在Tb级别以上。其中,华大智造的DNBSEQ-T20×2单次运行的通量可以达到42Tb(PE100)或72Tb(PE150),比常规超高通量测序仪的通量高出4.5倍至7倍,是全球最高通量的基因测序仪之一。
读长和速度方面,国产基因测序仪的表现同样亮眼。基因测序的读长,是指在一次测序反应中能够测得的DNA序列的长度。在二代测序中,单个DNA分子需要扩增成基因簇进行同步复制,以增强荧光信号强度从而读出DNA序列,因为读长较短,往往在50bp~300bp之间,而纳米孔测序技术获得的测序读长则相对较长。2023年以来,国内纳米孔测序仪市场也悄然爆发。其中,齐碳科技的QNome-3841hex可以产出测序读长在200bp~2Mbp,华大序风刚刚推出的CycloneSEQ-WT02,也可以达到Mbp级别的测序读长,在疾控、公安等领域的应用在陆续铺开。
测序速度相对直观,也是基因测序仪是否适合临床应用场景的重要指标。华大智造的DNBSEQ-G99,是目前全球中小通量测序仪中速度最快的机型之一,12小时可完成PE150测序,适用于小样本量的肿瘤靶向测序、小型全基因组测序、低深度WGS测序、个体识别、16s宏基因组测序等多种应用。而万众一心的MICRO-ATGC,用无磁珠测序法,利用电信号代替传统光学信号,实现高速电子测序。
再来看功能。基因测序仪厂商主要通过推出不同通量的机型,来覆盖广泛的应用场景。高通量机型主要用于服务大规模、大人群的基因测序需求,比如大人群的全基因组测序、超深度外显子组测序、肿瘤Panel测序等,往往需要用到高通量测序仪。中通量基因测序仪主要用在常规的科研实验室中,低通量基因测序仪的应用场景则主要在临床上,比如肿瘤和感染性疾病的靶向测序、肿瘤早筛、遗传性疾病的筛查等。在国内,华大智造、真迈生物、赛陆医疗都完成了覆盖高、中、低通量基因测序仪的产品构建,从而适应各种场景下的基因测序需求。
最后是技术平台。伴随着齐碳科技的QNome-3841、QNome-3841hex和华大序风的CycloneSEQ-WT02等机型的推出,国产基因测序仪所采用的核心技术,从此前主流的合成测序法向纳米孔测序法延伸,已经覆盖了各种主流的基因测序技术平台。
从某种意义上讲,在风起云涌的2024年,源自国内的基因测序仪,已经形成了为全球产品品类最丰富、功能最完善、技术最为多样化的基因测序仪市场,为应用生态的繁荣打下了基础。
夹缝中的增量市场,面临火拼
现阶段,基因测序仪的应用场景主要有两大类,即科研和临床。其中,科研服务仍然是基因测序仪最主要的应用场景,市场占比过半。不过,基因测序仪的科研服务市场已经相对成熟,增长速度也远不及临床创新带来的增量市场。尽管科研服务市场可能会被作为基因测序仪商业化应用的第一站,但真正产生足够大的商业价值,是在打开临床应用的大门以后。而临床检测创新这个夹缝中的增量市场,也成为国产基因测序仪厂商的兵家必争之地。
在国内,临床基因检测区别于血常规、肝肾功等常规检验项目,以特检项目的形式存在。而基因检测的商业模式有LDT和IVD两种类型。具体而言,LDT即实验室自建项目,是一种在实验室内部研发、验证和使用的体外诊断试剂提供检测服务的模式。这种检测方法通常用于临床诊断,但尚未获得产品注册或备案,仅限于研发实验室内部使用。而在IVD模式下,基因检测被作为常规的临床检测项目,由医院采购基因测序仪和对应的检测试剂,在院内展开。
尽管自《关于开展医疗机构自行研制使用体外诊断》的文件发布以来,LDT模式获得了特定情形下的合法身份,但由于渠道维护难度大、处方上量速度慢,LDT并不是基因检测在临床普及最理想的模式。大多数下游的基因测序服务商都在尝试将自家的基因检测项目批量送到医院内。
在这个过程中,除了取得相关的试剂注册资质,与专业的基因测序仪厂商合作,开发符合医疗器械要求的基因测序仪,成为大多数服务商的选择,也给国产基因测序仪厂商创造了重要的装机量提升机会。
而临床创新检测市场,也是不确定性最强的医疗市场之一。随时都有企业出局,也有新王出现。
目前,华大智造凭借领先的合作数量,暂具优势。据动脉网统计,目前,共有25款国产基因测序仪获得了三类医疗器械注册证,即可以搭配国家药监局批准的体外诊断试剂以及仪器配套随机软件,在临床上使用。其中,包括吉因加生物的Gene+eq-2000、微远基因的VisionSeq 1000在内,有16款国产基因测序采用了联合探针锚定聚合测序技术平台,也使得这项由国内团队持有的专利技术,成为国产基因测序仪最主流的技术平台。
联合探针锚定聚合测序技术由华大基因主导开发,其核心是使用DNA纳米球(DNB)进行模板扩增和信号放大,是通过设计多个特异性DNA探针来捕获并富集目标序列,然后利用荧光标记的核苷酸在DNA聚合酶的催化下,在DNA纳米球(DNB)上进行聚合,从而实现对目标序列的高通量测序。
联合探针锚定聚合测序技术优势明显,也是华大智造基因测序仪核心技术平台DNBSEQ的关键内核。DNBSEQ平台正是利用联合探针锚定聚合测序技术,将测序引物锚定分子和荧光探针在DNA纳米球上进行聚合,然后通过高分辨率成像系统采集光信号。华大智造的DNBSEQ-T7、DNBSEQ-G99等基因测序仪,都采用了联合探针锚定聚合测序技术,来提高测序效率和降低成本。
目前,华大智造的临床检测开发合作版图覆盖了大多数主流的下游服务厂商,先发优势明显,吉因加生物、泛生子、微远医疗、贝康医疗等在肿瘤伴随诊断、感染性疾病检测、辅助生物检测等基因测序的主流应用场景占有一定市场地位的服务商,都基于华大智造的技术平台开发自家的硬件产品。不过,考虑到以硬件+试剂模式落地院内的基因检测体量还十分有限,华大智造的这种先发优势带来的商业价值,还比较微弱。
值得注意的是,另一边,真迈生物则展现出了强大的兼容融合,有望快速进入成熟的临床基因检测场景中。具体而言,真迈生物自主开发的GenoCare 1600、GenoLab M Dx,分别采用了边合成边测序技术和可逆末端终止测序技术。其中,可逆末端终止测序是二代测序中最关键的底层技术。可逆末端终止测序的基本原理,是通过在DNA合成过程中引入可逆终止的荧光标记核苷酸,实现边合成边测序。可逆末端终止测序的专利原本属于Solexa,Illumina通过产业并购获得了这项专利,并进一步开发形成了后来风靡全球的边合成边测序(SBS)平台。
据报道,真迈生物的GenoLab M Dx,是首款国产自主知识产权的可逆末端终止测序法高通量基因测序仪,采用基于芯片扩增的表面荧光测序技术SURFSeq对碱基的荧光信号进行识别,实现边合成边测序,可兼容主流NGS建库试剂盒和生信分析软件。尽管真迈生物没有明确表示基于Illumina平台开发的试剂和软件可以在GenoLab M Dx上运行,但采用更主流的底层技术体系,无疑增加了GenoLab M Dx的适用场景。
此外,泛生子的GENETRON S5和达安基因的DA8600采用了半导体测序法,这种相对传统的基因测序技术,在今天的国内基因测序仪市场上,仍有一席之地。半导体测序法通过检测DNA聚合酶在合成过程中游离核苷酸引起的退火电流信号,来确定DNA序列,不需要荧光探针和昂贵的光学设备,可以显著降低测序成本。半导体测序技术主要应用在Ion Torrent系的基因测序仪上,伴随着Ion Torrent先后被Life Technologies和Thermo Fisher收购,半导体测序技术的核心专利,也落入Thermo Fisher手中。
由此可见,在临床创新检测这个夹缝中的增量市场上,无论是国产基因测序仪与海外巨头之间,还是在国产品牌内部,都还有许多的激战要拼。
谁将胜出?
在商业化拓展的过程中,基因测序仪与垂直应用领域的绑定极深。对基因测序仪厂商而言,得应用场景者,得天下。
仍以临床创新检测为例,基因测序仪往往与特定检测试剂和软件、特定疾病同时出现。而作为高值耐耗品,基因测序仪的周转周期长,基因测序试剂的销售状况,往往决定了基因测序仪厂商的经营业绩。根据2024年半年财报数据,华大智造的试剂耗材收入占比为60%,而Illumina的试剂耗材收入占比则超过70%。
从这个意义上讲,基因测序仪市场竞争的内核,拼的是厂商的应用生态构建能力。一方面,基因测序仪厂商要与下游的基因测序服务机构、药企等深入合作,推出基于其技术平台开发的临床检测试剂盒,把存量市场做大。
据动脉网统计,截至发稿,国内获批用于临床的基因检测试剂盒共有52个,覆盖肿瘤伴随诊断、生殖医学、感染疾病检测等领域。其中,基于可逆末端终止测序法开发的试剂盒22个、基于联合探针锚定聚合测序法开发的试剂盒10个、基于半导体测序法开发的试剂盒8个。结合前文分析,这背后的主要基因测序仪厂商分别是Illumina、华大智造和Thermo Fisher。Illumina的商业化优势十分明显,而真迈生物凭借系统兼容性,也有可能后来者居上。
实际上,与下游深度合作开发应用试剂,正是Illumina关键的商业化策略。尤其是在肿瘤伴随诊断领域,Illumina推动下游测序服务上与肿瘤靶向药厂商合作开发伴随诊断,实现了基因测序在肿瘤诊疗中的广泛应用。近年来,Illumina更是频繁亲自下场,参与开发肿瘤相关诊断试剂。其中,最著名的事件,莫过于Illunmina在Grail之上的反复投入,推动了多癌种早筛产品的上市。此外,2020年1月,Illumina还直接与罗氏制药合作,共同宣布建立一项长达15年的非排他合作关系,致力于拓宽基于新一代测序技术(NGS)开发的肿瘤检测方案的临床应用。
另一方面,基因测序仪厂商还需要通过线上论坛、线下会议等方式,来与更广泛的基因测序仪使用者合作,构建开发者生态,持续开发新的应用场景。在这方面,牛津纳米孔的探索颇为成功。通过Nanopore社区和London Calling,牛津纳米孔逐步打开了纳米孔测序仪在下游的应用,并且持续优化本身的测序能力。其中,Nanopore社区面向牛津纳米孔的所有用户开放,用户可以在其中获得实验支持,与其他用户协作,并且是新产品和更新的首批知情者。London Calling则是由牛津纳米孔技术主办的年度会议,吸引了来自全球的科学家和研究人员,他们在此交流利用纳米孔测序技术在微生物、宏基因组等多个领域的研究成果。
在国内,基因测序仪厂商与下游服务商的合作正变得更加紧密。华大智造本来就出身于国内基因测序服务龙头华大基因,这种产业链合作自不必说。在2021年1月,真迈生物也引入圣湘生物作为第二大股东,获得2.55亿元融资。圣湘生物将有权参与其重大经营决策。而从文章开头提到的新品发布会频繁举行看,国产基因测序仪厂商的生态构建意识也正转变为各种创新的尝试。
在夹缝中爆发的国产基因测序仪,能否在更广阔的应用场景中持续绽放,我们拭目以待。
*封面图片来源:123rf
如果您认同文章中的观点、信息,或想进一步讨论,请与我们联系;也可加入动脉网行业社群,结交更多志同道合的好友。
近
期
推
荐
声明:动脉网所刊载内容之知识产权为动脉网及相关权利人专属所有或持有。未经许可,禁止进行转载、摘编、复制及建立镜像等任何使用。
动脉网,未来医疗服务平台
核酸药物基因疗法
2024-08-14
·药渡
为促进创新药研发学术交流,助力创新药高质量研发,泛生子策划出品创新药物研发系列视频节目《药精准》,邀请创新药企大咖和研发团队负责人、临床及科研PI、监管方、创新生物技术专家等,围绕肿瘤靶向治疗的发展、肿瘤新药开发、诊断技术、医药法规政策等领域的科学内容进行分享和探讨,共同推动中国医药创新企业研发探索和行业思考。
《药精准》第23期
随着精准医学和分子诊断技术的发展,微小残留病灶(Minimal Residual Disease,MRD)的检测已成为一种重要的临床需求。作为MRD新兴检测技术—二代高通量测序方法(NGS)已受到国内外专家的认可和推荐,2022版《NCCN多发性骨髓瘤临床实践指南》、2021版《NCCN急性淋巴细胞白血病临床实践指南》等指南中均推荐了NGS作为MRD检测方法。
在此背景下,《药精准》系列直播【第23期】,聚焦“新兴MRD技术在血液肿瘤中的精准检测策略及临床进展”,并邀请到了北京大学人民医院 阮国瑞博士、浙江大学医学院附属第一医院 胡永仙教授、罗氏 李文锦博士以及艾沐蒽 范林洋博士几位临床专大咖和药企研发专家,一同探讨MRD检测技术在临床中的最新应用进展及推动精准诊疗的无限潜力。
8月15日19:00-20:30,我们云端相约!
欢迎扫码预约观看
嘉宾信息
阮国瑞
北京大学人民医院血研所
研究员
国家血液系统疾病临床医学研究中心PI
中国实验血液学杂志编委、Cancer Lett编委
国家药检局医疗器械技术审评中心外聘专家
国家自然科学基金、科技部、教育部、北京市科委等项目/成果评审专家,Leukemia、Molecular Diagnosis & Therapy、中华医学杂志(英文版)、中华医学会系列期刊等评审专家
主要从事恶性血液病分子标记物的基础与临床研究,主持/承担国家973计划、国家自然科学基金、教育部高校博士点基金、北京市自然科学基金等多项研究
在Molecular Cancer、Journal of Hematology & Oncology、Clin Cancer Res等杂志发表论文97篇
已授权专利30项,多项转化应用,并在血液肿瘤精准诊断、预后评估、微小残留病监测及治疗方案选择中发挥重要作用
曾获省部级科技进步奖、北大医学部发明专利奖等30余项
在国际上首先发现了十种新的血液肿瘤相关基因突变,并被美国国立生物技术信息中心收录
胡永仙
浙江大学医学院附属第一医院骨髓移植中心
主任医师
浙江大学教授、主任医师、博士生导师
擅长CAR-T细胞治疗难治复发急性淋巴细胞白血病,淋巴瘤和多发性骨髓瘤
浙江省卫生高层次创新人才
浙江大学医学院临床青年拔尖人才,浙江大学医学院创新团队培育项目学术带头人浙江大学医学院附属第一医院骨髓移植中心科副主任
现任中华医学会血液学分会淋巴细胞疾病学组委员,中国抗癌协会血液肿瘤专委会委员,中国研究型医院学会细胞研究与治疗专业委员会常务委员,Cell Transplantation杂志编委,国家自然科学基金通讯评审专家
近5年主持国家级及省部级项目5项,以第一或通讯作者(含共同)在New England Journal of Medicine、Nature、Lancet Haematology、Cell Research等期刊发表高水平论文50余篇
李文锦
罗氏中国生物标志物研发部血液肿瘤
负责人
毕业于上海交通大学Bio-X研究院,是交大和加州大学洛杉矶分校联合培养博士,现任罗氏中国生物标志物研发部血液病负责人,曾先后在安进中国以及百济转化医学部担任生物信息科学家的工作。
范林洋
杭州艾沐蒽
技术总监
北京理工大学生物医学工程博士,医疗器械高级工程师,杭州高层次人才(E类)
2021-2023在国家纳米科学中心做博士后研究。
Nature communication;analytical chemistry 等发表学术论文 10 篇,总影响因子超100分,申请发明专利6项
现任职艾沐蒽生物科技有限公司学术总监,10年来一致致力于医药检测方法相关研究,具体研究领域包括抗体,多肽,RNA等生物大分子检测方法的开发和临床应用,在CTC与B/T细胞受体检测有一些独到经验
报名方式
一、微信端观看
扫描下方二维码。
二、网页端观看
https://cast.263live.net/rpEef4 复制该网址并在浏览器中打开,填写完成报名者信息即可观看直播课程。
三、进群交流
细胞疗法信使RNA临床1期
2024-08-14
·药融圈
#创新药 #苏州 #8月15-16日 #抗体 #小核酸 #XDC #核药 #临床 #抗体 #多肽 #小分子 #早期开发 #靶向蛋白降解 #FDA #免疫疗法 #高校科研院所 #中美双报 #投资并购
在这个充满挑战与机遇的时代,我们深知每一位新药领域的创新者和创业者都在寻找突破自我、实现企业持续成长的路径。#2024生物医药创新博览将于8月15-16日在苏州召开,本次博览会策划了新药创新者穿越周期,看透波动的系列活动,由创新药CEO趋势闭门会,14个新药开发高峰论坛,苏州特色企业参观,新药创新者晚宴等类型的活动,旨在帮助创新者和创业者找到研发创新和企业发展的源动力。
PART.1
[ 大会信息 ]
会议地点 | 苏州国际博览中心B/C馆
会议时间 | 2024年8月15-16日
支持媒体 | 药融圈、药事纵横、药通社、生物药大时代、细胞基因治疗前沿、药鼎记、原料药情报局、药融云、Pharma CMC、临研人、星北·自贸壹号、触界生物
会议规模 | 15,000+人
展览面积 | 36,000+m2
PART.2
[ 话题框架 ]
PART.3
[ 会议亮点 ]
扫码报名参会
PART.4
[ 会议议程 ]
会议日程可能存在变动,以会议现场为准
8月15日 B馆B101 抗体掌门人对话
联合主办:壹生科、上海市生物信息学会智能药物专委会
会议日程
主持人:陈明久,博奥信创始人、董事长兼首席执行官
08:55-09:00 联合主办方致辞
马宁宁 | 沈阳药科大学,教授;壹生科,董事长&创始人
09:00-09:30 可变区定点偶联技术(OC-BODY)在单抗和双抗中的应用
马宁宁 | 沈阳药科大学,教授;壹生科,董事长&创始人
09:30-10:00 基于AI的抗体发现与设计
曹志伟 | 复旦大学,教授
10:00-10:30 ADC早期研发的生物学和DMPK评价
李黎 | 爱思益普生物,VP
10:30-11:00 复宏汉霖的全球化战略
盛严慈 | 复宏汉霖生物制药全球战略与项目管理总经理
11:00-12:00 圆桌:如何走出差异化生物科技企业出海之路?
主持人:马宁宁 | 沈阳药科大学,教授;壹生科,董事长&创始人
杨嘉明 | 丽珠生物医药有限公司,常务副总经理
高长寿 | 艾力斯医药,首席技术官
陈立模 | 女王之舟药业,董事长&CEO
滕毓敏 | 赛洱纳博生物,联合创始人&CEO吴其威 | 智享生物,CTO
华 烨 | 烨辉医药,董事长兼首席执行官
12:00-13:30 午餐
主持人:刘江海,成都盛世君联,CEO
13:30-14:00 抗病毒单克隆抗体药物研发进展
严景华 | 昌平实验室新型疫苗和抗体药物领域领衔科学家
14:00-14:30 HER2抗体偶联药物诱导间质性肺炎的生物学机制研究
郭 鹏 | 中科院杭州医学研究所,教授
14:30-15:00 抗体免疫学基础研究进展
孟飞龙 | 中科院分子细胞科学卓越创新中心,研究员
15:00-15:30 TBD
王春河 | 达石药业,董事长 &创始人
15:30-16:00 纳米抗体早研的案例分享和思考
刘江海| 成都盛世君联,CEO
8月16日 B馆B101 抗体掌门人对话
联合主办:壹生科、上海市生物信息学会智能药物专委会
会议日程
主持人:曹志伟,复旦大学,教授
09:00-09:20 研究者视角看ADC药物研发现状及问题
薛俊丽 | 上海东方医院,教授
09:20-09:40 ADC药物应用场景和抗体的选择
李元浩 | 荣昌生物,副总经理
09:400-10:00 双特异性纳米抗体介导的胞内蛋白靶向降解
马兴元 | 华东理工大学 教授
10:00-10:20 创新的抗体-细胞因子融合蛋白的研发
周海平 | 海徕科,CEO
10:20-10:40 高覆盖度人源膜蛋白兔单克隆抗体的制备及应用
王 平 | 致知生物,首席商务官
10:40-12:00 抗体偶联药物应用的临床进展
韦 青 | 浙江省肿瘤医院,胃胰肠肿瘤内科副主任医师
11:00-12:00 圆桌讨论:多种类型抗体相继出现,未来抗体的演进方向在哪里?
主持人:马宁宁 | 沈阳药科大学,教授;壹生科,董事长&创始人
李新芳 | 迈百瑞,CEO
陈明久 | 博奥信创始人、董事长兼首席执行官
翁志兵 | 三生制药,CDMO事业部总经理
杜 新 | 埃格林医药,联合创始人&CEO
董 欣 | 逻晟生物,创始人&董事长&CEO
段 清 | 东曜药业,药物研发及技术开发负责人
陈建新 | 臻格生物,董事长&CEO
8月15日 B馆B102 GLP-1及多肽论坛
会议日程
主持人:李湘,中肽生化创始人&董事长
09:00-09:25 多肽类药物在临床期间的CMC研究的多变性
苑字飞|甘李药业,副总经理,首席技术官
09:25-09:50 GLP-RAs新药研发趋势-长效化、多靶点
陈小新|众生睿创,CEO
09:50-10:15 创新型靶向GLP-1R/GIPR的双靶点激动剂的发现和临床转化
甘雨龙|道尔生物,临床运营高级总监
10:15-10:40 iCVETide多肽新药发现平台技术及实用案例分享
孟广鹏|成都诺和晟泰,创新药总监
10:40-11:05 多肽药物的质量控制策略
付玉清|健元医药,高级研发总监
11:05-11:30 GLP类药物研发进展及商业化前景
章 华|鸿运华宁,研发高级总监
11:30-11:55 GLP-1药物的差异化发展策略
谢 天|甘李药业,医学事业部负责人
11:55-12:20 圆桌对话:多肽治疗领域扩展及出海机会
主持人:李湘 | 中肽生化创始人&董事长
陈小新|众生睿创,CEO
孙汉栋|九源基因,副总经理
徐文洁 | 银诺医药,高级副总裁&首席商务官
徐红岩 | 吉尔多肽,董事长
12:00-13:30 午餐
主持人:赖锦昌,美迪高,总经理
13:30-13:55 GLP-1,除了减重,还能卷什么?
左亚军 | 仁会生物,总经理
13:55-14:20 司美格鲁肽与替尔泊肽杂质鉴定研究案例分享
刘国柱 | 长沙晨辰医药,创始人&董事长
14:20-14:45 新一代人源超长效GLP-1药物创新研发之路及关键临床数据解读
徐文洁|银诺医药,高级副总裁&首席商务官
14:45-15:10 多肽实体文库高通量筛选体系及特色应用开发
林 定|禾泰健宇生物,研发总监
15:10-15:35 伊匹乌肽-替代糖皮质甾体激素的广谱抗炎原创药
夏献民|益承生物,董事长&总经理
15:35-16:00 EMPHASES多肽合成技术及在药物多肽合成中的应用
郑庆泉|同隽医药,创始人&董事长
16:00-16:25 环肽创新药的发现:从长效棘白菌素到口服PCSK9抑制剂
原晨光|祥根生物,联合创始人&CSO
8月16日 B馆B102 核酸药物开发前沿论坛
会议日程
主持人:冯冰,中肽生化,寡核苷酸研发资深总监
09:00-09:20 mRNA疫苗与mRNA药物
回爱民 | 惠正奇医药,创始人
09:20-09:40 靶向mRNA的碱基编辑技术开发与应用进展
忻 蓉 | 时夕生物,总经理
09:40-10:00 小核酸药物的临床前药代动力学研究
刘文玥 | 华西海圻,药代技术总监
10:00-10:20 小核酸干扰药物的递送和临床进展
张佩琢 | 吉玛基因,董事长
10:20-10:40 寡核苷酸药物中的杂质来源
冯 冰 | 中肽生化,寡核苷酸研发资深总监
10:40-11:00 小核酸药物的创新实践
苏晓晔 | 石药集团,核酸药物研究院负责人
11:00-11:20 Nucleosidic phosphoramidites: an optimized approach in their synthesis, quality evaluation and impurities characterization
Beatrice Trucchi | Flamma S.p.A. - Italy R&D Project Manager
11:20-12:00 BH-RACETM新型核酸药物平台
胡 翰 | 滨会生物,副总裁
12:00-13:30 午餐
主持人:张力,北京瑞博奥医药科技有限公司,副总经理
13:30-13:50 寡核苷酸药物的DMPK的研究挑战和案例分享
陈桂英 | 宏韧医药,化学药物生物分析部门总监
13:50-14:10 治疗肝和肺部疾病的siRNA全新分子设计
黄 毅 | 国为医药,研究院副院长
14:10-14:30 全和诚核酸药物递送平台(拟定,以现场为准)
周经经 | 全和诚,研发副总经理
14:30-14:50 激发小核酸长效性潜力开发慢性病案例(拟定,以现场为准)
万金桥 | 先衍生物,总经理
14:50-15:10 AI驱动的高效mRNA疫苗开发
费才溢 | 澄实生物,vp&联合创始人
15:10-15:30 双靶向核酸适体技术
王雪强 | 中国科学院杭州医学研究所,研究员
15:30-15:50 小核酸干扰药物的递送和临床进展
袁旭东 | 艾康药业,CEO
8月15日 B馆B103 XDC领域的差异化创新和国际化策略
大会主席:夏明德,英诺湖医药,CEO&董事长
会议日程
主持人:夏明德,英诺湖医药,CEO&董事长
08:55-09:00 大会主席致辞
夏明德|英诺湖医药,CEO&董事长
09:00-09:20 ADC FIC 药物因CMC的生产缺陷导致批准上市延缓的启示
刘翠华|百奥泰生物,高级副总裁
09:20-09:40 小型多肽类及双靶向偶联药物的研究、临床开发进展和成药展望
邵 军|同宜医药,首席技术官
09:40-10:00 宜联生物TMALIN ADC技术平台:提高药效、在新空间寻找靶
蔡家强|宜联生物,联合创始人&CSO
10:00-10:20 MIDD视角下的ADC药物开发
苏 霞|康龙化成,临床药理&定量药理高级总监
10:20-10:40 未来已来:端到端的创新ADC药物发现与商业化开发模式
秦 刚|启德医药,CEO
10:40-11:00 核药临床试验设计的特殊性与实施的挑战
刘 艳|米度生物,首席医学官
11:00-11:20 ADC的组合创新
谢雨礼|上海微境生物,创始人&总经理
11:20-12:00 圆桌:如何从早期设计降低XDC的临床失败?
1.XDC从早期设计到临床开发成败案例
2.失败的原因分析
3.从失败的案例中可以得到哪些启发?如何避免
主持人:夏明德|英诺湖医药,CEO&董事长
刘翠华|百奥泰生物高级副总裁
邵 军|同宜医药,COO
蔡家强|宜联生物,CSO
秦 刚|启德医药,CEO
肖志华|奥浦迈,董事长
1200-1330 午餐
主持人:刘艳,米度生物,首席医学官
13:30-13:50 生命科学前沿应用之ADC药物研发
徐雍羽|瑞孚迪,类器官和生物制药项目经理
13:50-14:10 New Drug Modality在未被满足临床上的探索--放射性偶联药物
单 波|博锐创合 ,CEO
14:10-14:30 创新核药研发与产业链之诺宇解决方案
方 鹏|诺宇医药,董事长&创始人
14:30-14:50 靶向Nectin4核素偶联药物的开发
习 宁|范恩柯尔,创始人&CEO
14:50-15:10 核药产业发展现状
邓启民|成都云克药业,总经理
15:10-15:30 靶向GPC3放射性药物的筛选与开发
宋 征|艾博兹医药,临床前开发副总裁
15:30-15:50 核素药物全球化专利布局挑战与策略
唐华东|植德律所,合伙人
8月16日 B馆B103 创新药早期开发
策划人&主持人:张水华,新樾生物,副总经理
会议日程
09:00-09:25 铁死亡研究的突破与新疗法的涌现
徐 峻 | 中山大学,教授
09:25-09:50 Smart DEL(DEL及AI相结合的)的筛选及案例分享
金 锋 | 新樾生物,副总经理
09:50-10:15 Explore New DEL Modality Beyond Binding Assay
黄已然 | 广东省小分子新药创新中心,新药筛选中心副总监
10:15-10:40 创新药靶点选择逻辑及临床前成药性分析--- From BD Perspective
韩奇峰 | 勃林格殷格翰,全球业务拓展及许可部门副总监
10:40-11:05 创新药靶点选择逻辑及临床前成药性分析--- From BD Perspective
吴宏忠 | 分子之心,商务副总裁
11:05-11:30 创新药立项评估策略
郑晓鹤 | 海正药业,创新药研究院副院长
11:30-12:15 圆桌对话:药物早期研发阶段的主要目标是什么?什么样的早期药物研发是成功的?
贺 耘 | 深圳湾实验室百瑞创新中心主任(主持人)
胡 畏 | 礼来中国,创新合作中心资深总监
边 峰 | BMS,全球药物研发中国综合科学团队执行总监
方剑武 | 大睿生物,副总裁
吴宏忠 | 分子之心,商务副总裁
12:15-13:30 午餐
【靶向蛋白降解药物开发】
策划人&主持人:曾雳,和径医药首席执行官
13:30-13:55 万物皆可降:靶向蛋白降解药物研发思路探讨
冯 焱 | 领泰生物,CEO
13:55-14:20 蛋白降解GlueTacs®平台构建及其肿瘤及免疫疾病治疗领域的药物开发
杨小宝 | 标新生物,创始人、董事长兼首席执行官
14:20-14:45 Bi-XDC技术及临床研究进展
王贵涛 | 同宜医药副总经理、研究院院长
14:45-15:10 分子伴侣介导的蛋白降解技术平台
英伟文 | 珃诺生物创始人兼首席执行官
15:10-15:35 靶向EGFR的PROTAC作为新型非小细胞肺癌的治疗方式
曾雳 | 和径医药,首席执行官
15:35-16:15 圆桌讨论:蛋白降解技术的技术开发趋势和挑战?
主持人:曾 雳 | 和径医药首席执行官
冯 焱 | 领泰生物,CEO
杨小宝 | 标新生物创始人、董事长兼首席执行官
英伟文 | 珃诺生物创始人兼首席执行官
胡逸民 | 胡逸民 Chimergen Tx ,联合创始人兼CSO
8月15日 B馆B106 新质生产力时代,国有企业与Biotech公司如何合作创新?
策划人:蔡学钧,前BMS,全球研发副总裁
会议日程
10:00-10:30 当前形势下,国企和Biotech面临的挑战和机遇
蔡学钧 | 前BMS,全球研发副总裁
10:30-10:50 新质生产力时代,国企该如何释放创新潜力和合作机会
10:50-12:00 圆桌对话:政策激励下,国企及Biotech各自的机遇和挑战
密集生物医药"真"创新政策鼓励下,国企该如何利用好政策优势聚集创新资源要素,Biotech公司应该如何与国企合作将创新资源要素转化成加速创新药落地的动力,解决当前行业寒冬下融资难、运营成本高的问题
叶 峰 | 创胜集团公司,首席运营官
12:00-13:30 午餐
13:30-15:30 自由交流:国企和Biotech如何构架价值闭环实现共同增长及项目合作机会探讨
新时代,国企如何起到排头兵作用,如何做好自己,如何构建合作实现价值闭环模型,如何实现投资回报,如何激发国企的创新潜力;Biotech公司如何借助国企在资本、生产、政策、流通等要素的优势加速创新从实验室走向市场,提升国际技术竞争力
崔兴品 | 华润医药集团,副总裁、华润三九医药董事
15:30-15:50 论坛总结发言
蔡学钧 | 前BMS,全球研发副总裁
8月16日 B馆B106 以临床转化为导向的临床前药效评价技术与经验分享
主办单位:格林泰科&药融圈
协办单位:BioBAY&艾名医学
会议日程
09:00-09:05 主办方开场
刘 婷 | 格林泰科,市场部
09:05-09:30 体外药效活性筛选技术在新药成药性研究和临床转化的作用
杨晓东 | 格林泰科,新药筛选平台负责人
09:30-09:55 类器官模型助力新药研发
周 轶 | 艾名医学,首席运营官
09:55-10:20 镇痛药物的开发现状及临床前药效评价方法
石 刚 | 格林泰科,副总经理/市场项目部总监
10:20-10:45 肿瘤治疗性疫苗非临床安全性评价策略
夏玉叶 | 苏州华测,机构负责人/副总经理
10:45-11:10 心血管系统疾病药物开发的动物模型选择
孙 艳 | 格林泰科,高级项目经理
11:10-11:35 帕金森药物的研发现状及临床前研究案例
江万祥 | 格林泰科,副总经理/机构负责人
11:35-12:00 医疗器械的大动物有效性和安全性评价经验分享
李扶慧 | 格林泰科,医疗器械有效性评价负责人
8月15日 C馆C101 FDA对新兴医疗产品的监管
联合主办:FDA专家协会
会议日程
主持人:李宁,君实生物,副董事长
09:00-09:05 致辞
李 宁|君实生物,副董事长
09:05-09:20 新兴医疗产品的概述
杜 新|埃格林医药,联合创始人&CEO
09:20-09:45 新兴医疗产品的发展趋势和监管挑战
陆金华|星尘生物,CSO
09:45-10:10 CGT 产品临床试验的注意要点
门宇欣|海昶生物,首席医学官
10:10-10:35 CGT产品的临床药理学考量
杨 劲|中国药科大学,教授
10:35-11:00 CGT产品的cGMP合规考量
孙志刚|绿叶制药集团,高级副总裁
11:00-11:25 cGMP合规考量下除菌过滤验证的挑战
杨潇军|挪亚泰尔,技术负责人
11:25-12:00 小组讨论:新兴医疗产品的国际化
李 宁|君实生物,副董事长
杜 新|埃格林医药,联合创始人&CEO
张明东|苏州赛纳思医疗,CMO
王幼民|银泉生物制药咨询有限公司,创始人&首席顾问
孙 鹤|天士力控股集团,全球副总裁
胡育志|香港白橡树,CEO
12:00-13:30 午餐
主持人:陈少羽, 美国安诺波特律师事务所驻上海代表处,管理合伙人
13:30-14:00 小核酸药物的开发与监管考量
杨永胜|海昶生物,高级副总裁&核酸创新研究院院长
14:00-14:30 FDA对溶瘤病毒产品的的监管
张 静|杰科生物,CMO
14:30-15:00 首个DMD基因疗法的FDA审评审批争议-案例研究
王亚宁|瑞宁康生物医药,创始人&CEO
15:00-15:30 美国食品药品监督管理局在药品研发中使用人工智能和机器学习的监管概况
韩晓梅|E7 CAPITAL,创始人&CEO
15:30-16:00 人工智能/机器学习创新医疗器械监管策略
胡云富|泛生子,首席医疗官
16:00-16:45 小组讨论:新兴医疗产品监管的进一步深化
陈少羽|美国安诺波特律师事务所驻上海代表处,管理合伙人
陆金华|星尘生物,CSO
韩晓梅|E7 CAPITAL,创始人&CEO
窦金辉|挚盟医药,注册法规事务副总裁
王亚宁|瑞宁康生物医药,创始人&CEO
8月16日 C馆C101 免疫治疗新药开发论坛
策划人&主持人:张立刚,冠科生物,中国商务区总经理
会议日程
主持人:张立刚,冠科生物,中国区商务总经理
09:00-09:20 转化医学研究协助HX009 CD47/PD-1双功能抗体的临床开发
李其翔 | 翰思生物,联合创始人&总裁和首席科学官
09:20-09:40 免疫疗法:双抗vs细胞治疗
顾津明 | 传奇生物,前中国区研发负责人
09:40-10:00 ImmunoCytokine: The New Era of Cancer Immunotherapy
殷刘松 | 盛禾生物,CEO&CSO
10:00-10:20 Regulatory T Cells and Personalized Immunotherapy
李 斌 | 上海交通大学,特聘教授、上海市免疫学研究所科研副所长
10:20-10:40 全新型双抗巨噬细胞衔接器用于癌症免疫疗法
卢宏韬 | 科望医药,联合创始人兼首席科学官
10:40-11:00 动物模型在I/O药物开发中的应用
王晶晶 | 冠科生物,太仓公司总经理
11:00-11:40 圆桌讨论:从2024国际会议看全球免疫疗法治疗趋势和机会
主持人:李佳 | 朗盛投资,合伙人
李丹 | 博亚方洲创投,副总经理
易琳 | 德福资本,合伙人
时永灵 | 聚明创投,合伙人
宁高见 | 元生创投,董事总经理
11:40-13:30 午餐
主持人:殷文劼,亘喜生物,药理毒理部高级总监
13:30-13:50 JAK1抑制剂在自免领域的应用
姜 非 | 康哲药业,大中华区首席投资官
13:50-14:10 双特异性抗体药物项目的开发进展
吴辰冰 | 岸迈生物,CEO
14:10-14:30 用于肿瘤免疫治疗的新型腺苷受体A2aR/A2bR拮抗剂的发现
王永辉 | 复旦大学讲座教授、励缔医药联合创始人
14:30-14:50 异体CAR-T药物开发进展
殷文劼 | 亘喜生物,药理毒理部高级总监
14:50-15:10 应用肿瘤类器官技术发现临床候选化合物
王 鹏 | 冠科生物,体外研发执行总监
15:10-15:30 胰腺癌个性化多肽
陈海敏 | 安达生物,COO
8月15日 C馆C102 新药创始人峰会
会议日程
主持人:邓永奇,凯复医药,CEO
09:00-09:25 如何应对复杂的生物医药产业环境?
王兴利|复星医药,执行总裁&创新药事业部联席CEO
09:25-09:50 华润医药在生物医药领域打造新质生产力的探索和思考
崔兴品|华润医药集团,副总裁、华润三九医药董事
09:50-10:15 绕不开的商业化,Biotech公司该怎么看待商业化?
刘 谦|阿斯利康中国,副总裁
10:15-10:40 当前市场环境下生物医药初创公司的发展策略
刘为民|百济神州广州创新中心生科创投,董事总经理
10:40-11:05 中国生物医药产业投资机会分析
毛 化|沙利文 ,大中华区合伙人兼董事总经理
11:05-12:00 圆桌:中国Biotech公司未来突破点有哪些?
1.被MNC并购,前提是准备好没?
2.进入集采,创新药的想象力真的那么好?
3.被国内大药企并购退出?
4.抓住机会从Biotech到Biopharm?
5.以仿养创?怎么养?
6.兼顾CRO业务获得现金流?卷得动吗?
主持人:邓永奇|凯复医药,CEO
刘为民|百济神州,广州创新中心生科创投,董事总经理
金 方|健康元,首席科学家
刘 谦|阿斯利康中国,副总裁
吴世斌|万邦医药,董事长
秦锁富|科兴生物制药,副总经理兼医药研究院院⻓
12:00-13:30 午餐
【中国新药的理性发展方向】
论坛策划人&主持人:
党 群,真实生物,总裁
13:30-13:55 创新药是选FIC还是BIC?解决临床未满足需要是关键!
党 群|真实生物,总裁
13:55-14:20 和誉医药FGFR抑制剂开发进展
陈 椎|和誉医药,联合创始人兼CSO
14:20-14:45 CAR-T治疗实体瘤的屏障与异体通用之路及研究实践
章登吉|美迪西,生物技术药物分析部负责人
14:45-15:10 YAP/TEAD抑制剂的开发
朱继东|奕拓医药,CEO
15:10-15:35 浅谈“前药”的前世今生-长乘医药的前药研发
宋钦辉|长乘医药,CMO
15:35-16:00 Delivery of Excellence - 递送引导的药物创新
沈 旺|维眸生物,创始人、董事长兼CEO
8月16日 C馆C102 抄底并购元年:中国Biotech的新出路、新活法、新征程
会议日程
主持人:陈锋,科林利康,副总裁/博羚资本,负责人
9:00-9:05 联合主办方致辞
陈 锋|科林利康,副总裁/博羚资本,负责人
09:05-09:35 2024全球生物医药License/并购趋势与策略
胡奇聪|波士顿咨询(BCG)合伙人、董事总经理
09:35-10:45 圆桌讨论:MNC如何与中国药企创新合作与双向奔赴
1.MNC的中国区战略布局和重点关注领域
2.MNC所理解的创新是什么
3.Biotech公司的管线没有进入MNC的list,如何进行策略调整?
4.创始人团队是否可以质押一些股权换得继续推进临床的资金?
5.恒瑞和康诺亚近期的出海案例,揭示了什么?
6.除此之外,还有哪些形式可以助力Biotech出海?
主持人:夏明德|英诺湖医药,董事长&CEO
陈 锋|科林利康,副总裁/博羚资本,负责人 王 昕|施维雅,全球业务拓展亚太区域总监 陈 晨|麦科奥特,首席商务官
蔡家强|宜联生物,CSO
10:45-12:00 圆桌讨论:穿越寒冬,中国本土Biotech的BD策略思考与交易前景分析
1.中国的新锐Biotech,如何立项有助于后续的BD合作开展
2.寒冬之下,中国Biotech的BD策略还有哪些值得优化的地方
3.下一个5年,中国创新药企的外部BD合作前景如何
主持人:金家齐|纽尔利基金,执行董事
胡会国|迈威生物董事、CBO、高级副总裁、董秘
姜 非|康哲药业首席投资官(大中华区)
杨建国|近邻生物CEO
12:00-13:30 午餐
13:30-13:50 致力于自主研发国际领先(FIC、BIC)的创新药物研发公司
王 喆|长森药业,创始人
13:50-14:10 创新抗板药物,提升心脑血管病患者治疗药效和安全性
陈小五|柯君医药化学副总裁
14:10-14:30 专注于细胞和基因治疗领域的生物研发赋能平台
夏珏妤|拓弘康恒,副总经理&首席战略发展官
14:30-14:50 为全球抗生素耐药问题提供差异化解决方案
邓一军|MetCura,创始人&CSO
14:50-15:10 工程化红细胞治疗自身免疫性疾病研究进展
吴岚林|科镁信生物,CEO
15:10-15:30 基于蛋白质动态构象技术平台开发的新一代KCNQ2/3激动剂
叶阳亮 |柱广生物,总经理
15:30-15:50 NuRapid® 新一代分子诊断技术产业化平台
何林富|为真生物,副总裁、CTO
15:50-16:10 AI在高效发现临床候选药物中的应用
邢 莉|朗睿生物医药,创始人/CEO
16:10-16:30 全球首个基于BCMA/CD19双靶并联的CAR-T细胞疗法的研发进展
蔡祥海|科弈药业,研发副总裁
16:30-16:50 做出国际首创新药,救治全球心梗病人
魏寅祥|永心生物,项目总监
8月15日 C馆C103 临床开发论坛
大会主席:杨修诰,石药集团,高级医学总监
会议日程
主持人:孟杰天,安徽万邦,首席医学官
09:00-09:25 从临床角度看中枢神经系统药物的临床开发策略及案例分享
申华琼 | 纽欧申医药,创始人&CEO
09:25-09:50 新药临床研发的适应症选择策略
朱永红 | 岸迈生物,CMO
09:50-10:15 关键临床试验失败案例分析
陈 霞 | 泰格医药,高级副总裁&首席医学官
10:15-10:40 抗体偶联药物(ADCs)的剂量优化相关临床研究及安全管理措施分析
杨修诰 | 石药集团,高级医学总监
10:40-11:05 从临床角度看ADC开发的挑战和前景
石 燕 | 启德医药,首席医学官
11:05-11:30 中国制造,出海有多难?
杜一鸣 | 海和药物,高级副总裁
11:30-12:10 圆桌:中国创新药注册及临床全球化操作技术的短板?
杨修诰 | 石药集团,高级医学总监(主持人)
刘艳玮 | 武田中国,副总裁 ,武田大中华区注册事务部负责人
杜一鸣 | 海和药物,高级副总裁
石 燕 | 启德医药 首席医学官
12:10-13:30 午餐
主持人:苏泉宇,一临云联合创始人&首席商务官
13:30-13:55 从临床视角看抗肿瘤创新药的临床开发策略及案例分享
隋 红 | 浙江瀛康生物医药有限公司,医学总监
13:55-14:20 神经退行性疾病药物发展:从过去看未来
陈柏州 | 加立生科,CEO
14:20-14:45 新药研发早期的统计方法
赵 萌 | 苏州信诺维,统计及编程执行总监
14:45-15:10 司美格鲁肽临床开发策略及要点
孟杰天 | 安徽万邦,首席医学官(CMO)
15:10-15:35 以终为始-数据分析驱动,提高创新药临床开发成功率
戴鲁燕 | 粹羽咨询,创始人
8月16日 C馆C103 临床开发论坛
大会主席:郑航,重庆医科大学,教授
会议日程
【临床试验创新设计与高质量发展】
主持人:郑航,重庆医科大学药学院,教授
09:00-09:30 新质生产力引领临床试验高质量发展
周 焕 | 中国药理学会药物临床试验专委会青年主任委员
09:30-09:55 临床试验数字化的现状与未来
郑 航 | 重庆医科大学药学院,教授
09:55-10:20 基因治疗产品的早期临床研究及案例分享
丁 珊 | 天泽云泰,高级医学总监
10:20-10:45 临床研究创新设计类型与统计实施
汤在祥 | 苏州大学,教授
10:45-11:10 抗肿瘤药物临床试验中的终点选择
任以中 | 葆元医药,医学事务高级总监
11:10-11:30 中国临床试验出海的机遇与挑战
邓晓宇 | 希毅医学,创始人/总经理
11:30-11:50 真实世界数据在临床研究中的应用
胡 皓 | 医数康成,总经理
11:50-13:30 午餐
13:30-14:00 监管科学视角下临床研究设计和质量的重大意义
侯 艳 | 北京大学,生物统计系研究员
14:00-14:30 我国药械组合产品监管新进展
许 伟 | 国家药品监督管理局医疗器械技术审评中心原副主任
14:30-15:00 数字化助力监管决策——面向药品现代化监管的智能化服务平台
王维玉 | 北京灵迅医药科技公司,联合创始人
15:00-15:20 从患者角度出发看DCT临床实践的融合与落地
夏素琴 | 创达医药,总经理
15:20-15:40 临床试验的“质”与“量”的博弈 — 付出越大,环节越多,质量就越好吗?
张 淼 | 南京麦普斯医药科技有限公司,创始人
15:40-16:00 临床试验不良事件的损害赔偿与风险管理
刘亚卿 | 上海浦东五新保险经纪有限公司,副总经理
8月15日 C馆C104 小分子药物开发策略
会议日程
主持人:李丽艳,默克中国,数字化产品经理
09:00-09:20 一种新型的基于分子胶payload的小分子偶联药物开发
任志华 | 开拓药业,VP/新药研究院院长
09:20-09:40 无人实验室技术及全新的化学实验工作模式
乔 木 | 融域智慧&云合成,总经理
09:40-10:00 AI加速化学合成的趋势
李丽艳 | 默克中国,数字化产品经理
10:00-10:20 中国创新药的困境、挑战和转型
陈新海 | 思康睿奇,化学VP
10:20-10:40 AI+自动化赋能新药研发
徐 涛 | 智化科技,副总裁
10:40-11:00 利用晶型技术评估和提高化合物的可开发性
徐 俊 | 晶云药物,研发高级总监
11:00-11:20 AI计算驱动的变构小分子激动剂开发
沈倩诚 | 宇道生物,CEO
11:40-13:30 午餐
13:30-13:50 Rediscovery of Paclitaxel: An innovative oral Paclitaxel softgel capsule product for improving cancer therapeutics
黄慧瑜 | 美济生物,首席科学官
13:50-14:10 怎么做好创新药研发风险评估?
陈 洪 | 外专局特聘专家
14:10-14:30 痕量杂质研究案例分享与高难度杂质研究平台
冯胜昔 | 艾奇西,总经理
14:30-14:50 神经系统疾病药物临床前药理研究要点
江万祥 | 格林泰科,副总经理/机构负责人
14:50-15:10 药物可开发性评估和处方前研究
唐开勇 | 泓博智源,副总裁
15:10-15:30 亚硝胺药物相关杂质的风险评估和控制策略考量
曹煜东 | 德睿智药,CMC 负责人
15:30-15:50 消化道疾病及消化道肿瘤免疫创新药开发
胡平生 | 贵州生诺生物,CEO
8月16日 C馆C104 高校科研院所生物医药转化论坛
论坛大会主席:罗有福,四川大学,教授
论坛副主席:叶庭洪,四川大学,教授
论坛秘书长:欧阳亮,四川大学,教授
会议日程
主持人:罗有福,四川大学,教授
09:00-09:25 细菌耐药性与抗菌药物研发
杨玉社 | 上海药物研究所,研究员
09:25-09:50 靶向线粒体ClpP的创新药物研发
罗有福 | 四川大学,博士生导师
09:50-10:15 基于未满足的临床需求如何优化新药分子设计
钱 海 | 中国药科大学,理学院院长
10:15-10:40 靶向RNA可变剪接抗肿瘤药物研发
张红河 | 浙江大学,基础医学创新研究院副院长
10:40-11:05 神经退行性疾病新靶点和先导小分子发现
欧阳亮 | 四川大学,教授
11:05-11:30 蛋白激酶抑制剂研究:强弩之末,还是正当其时?
张 健 | 上海交通大学,教授/宇道生物创始人
11:30-11:55 抗纤维化药物转化研究
叶庭洪 | 四川大学,博士生导师
11:55-13:30 午餐
13:30-13:55 靶向电子传递链QcrB以及直接靶向InhA的抗分枝杆菌新药研发
张天宇 | 中国科学院广州生物医药与健康研究院(GIBH),感染与免疫研究中心副主任
13:55-14:20 抗耐药真菌新技术、新靶点和新先导物的发现及转化研究
张大志 | 海军军医大学,教授
14:20-14:45 靶向蛋白降解药物的基础研究和转化
董晓武 | 浙江大学,教授
14:45-15:10 聚焦合成致死2.0策略,为MTAP缺失的肿瘤提供全方位的创新解决方案
陈旭星 | 优理生物,创始人&首席执行官
15:10-15:35 巨噬细胞干预纳米药物用于消化道肿瘤治疗
刘祥瑞 | 浙江大学医学院,教授/奥睿德医药创始人
15:35-16:00 基于脑肠轴的多发性硬化发病机制研究与干预
周嘉伟 | 中国科学院脑科学与智能技术卓越创新中心/神经科学研究所,研究员
8月15日 C馆C105 创新药中美双报如何制定研发策略
策划人:邹灵龙,康维讯生物,创始人、董事长&CEO
会议日程
主持人:邹灵龙,康维讯生物,创始人、董事长&CEO
09:00-09:30 创新TCE技术降低CRS风险提升成药潜力
曹国帅 | 天港医诺,研发负责人
09:30-10:00 Strategies for accelerating the time and reducing the cost of orphan drug global development in gene therapy
Alvin Luk | 辉大基因,联合创始人&首席执行官
10:00-10:30 支持ADC出海的ADME研究策略,方法和实例
朱明社 | 苏州锐迪欧,首席科学官&首席顾问
10:30-11:00 中美申报CMC要点
Audrey Jia | Wonderfulland LLC,CEO;前FDA CMC 审评员
11:00-11:30 中美双报生物分析策略与实操分享
邹灵龙 | 康维讯生物,创始人、董事长&CEO
11:30-13:30 午餐
主持人:曾勇,华仁医学科技集团,集团总裁
13:30-14:00 Immunogenicity Testing Strategy & Regulatory Expectation on Method Validation- FDA perspective
严皓珩 | 复宏汉霖,美国注册负责人,前FDA评审
14:00-14:30 出海申报的临床试验设计与数据管理
杨修诰 | 石药集团,高级医学总监
14:30-15:00 中美IND申请非临床研究要点
贺全仁 | 艾博生物,药理毒理高级副总裁
15:00-15:30 中美药品电子申报 (eCTD)要点
任 翀 | 复宏汉霖,药政注册副总监
@趋势@合作@学习@社交@参观扫码报名参会
PART.5
[ 往届会议 ]
点击下方链接可查看往届会议日程:
第二届中国国际生物&化学制药博览会(CMC-China)
第三届中国国际生物&化学制药博览会(CMC-China)
第四届中国国际生物&化学制药博览会(CMC-China)
第五届中国国际生物&化学制药博览会(CMC-China)
并购免疫疗法疫苗抗体药物偶联物
100 项与 北京泛生子生物科技有限公司 相关的药物交易
登录后查看更多信息
100 项与 北京泛生子生物科技有限公司 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
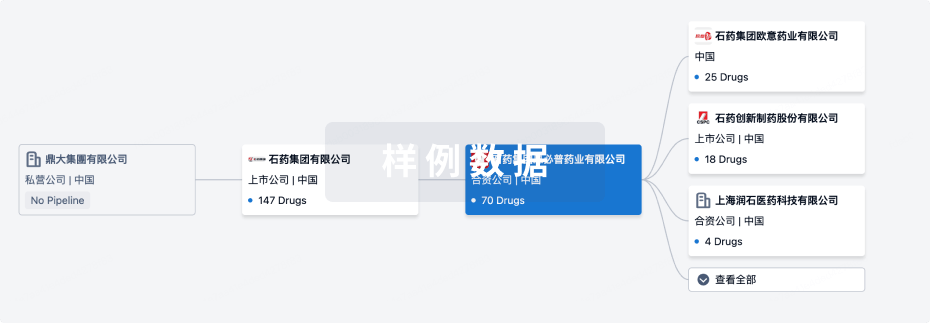
管线布局
2024年11月17日管线快照
无数据报导
登录后保持更新
药物交易
使用我们的药物交易数据加速您的研究。
登录
或

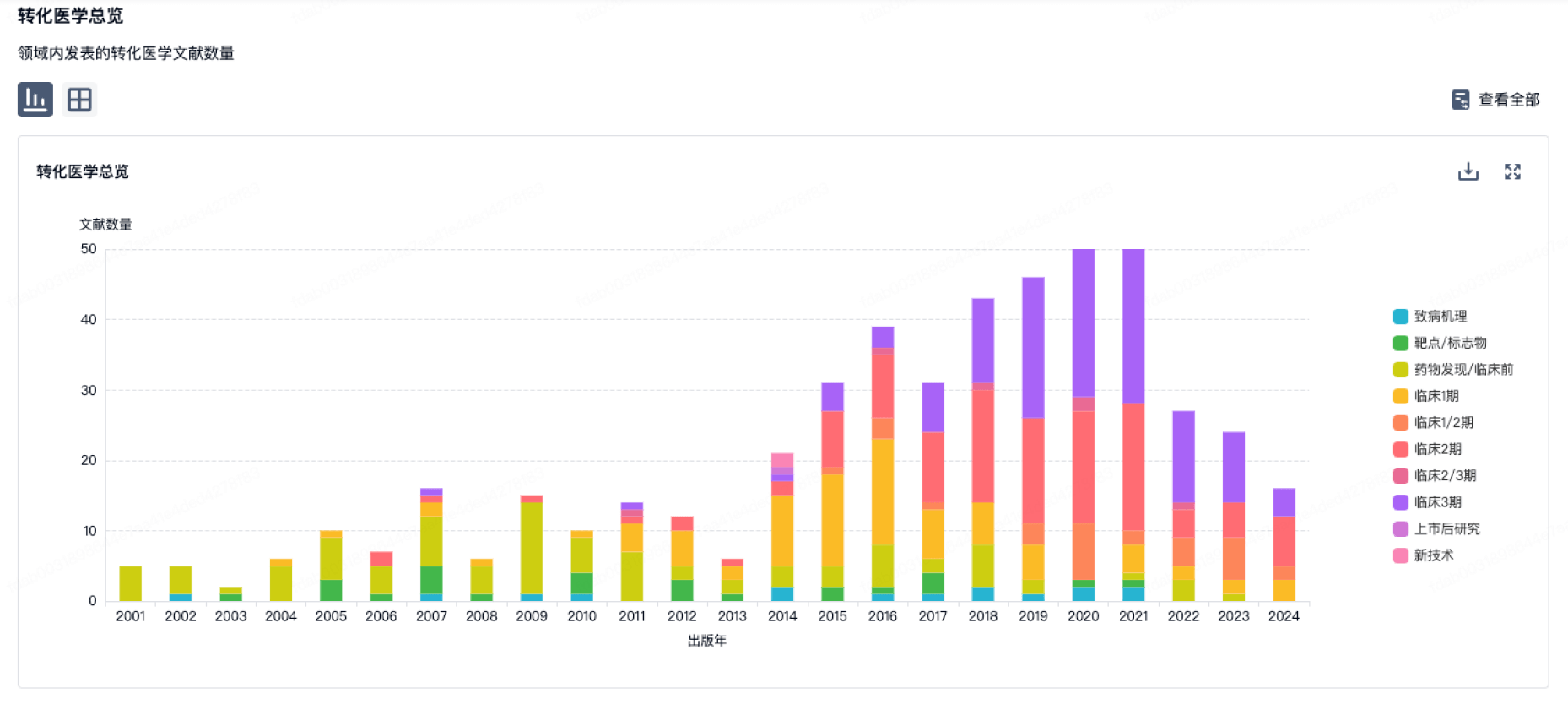
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或

投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或

融资
发掘融资趋势以验证和推进您的投资机会。
登录
或

标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用