更新于:2024-11-01
Green Cross Laboratories
更新于:2024-11-01
概览
关联
100 项与 Green Cross Laboratories 相关的临床结果
登录后查看更多信息
0 项与 Green Cross Laboratories 相关的专利(医药)
登录后查看更多信息
140
项与 Green Cross Laboratories 相关的文献(医药)2024-11-01·Annals of Laboratory Medicine
Effect of Blood Collection Tubes on Vitamin D Immunoassay Results
Letter
作者: Lee, Sangyoon ; Chae, Hyojin ; Cho, Sung-Eun ; Oh, Eun-Jee ; Choi, Ae-Ran
2024-10-01·Laboratory Medicine Online
A Survey on the Utilization and Quality Management of Handy Tests or Point-of-Care In Vitro Diagnostic Tests in Korea
作者: Park, Hyung-Doo ; Yoo, Soo Jin ; Chun, Sail ; Um, Tae Hyun ; Choi, Rihwa
2024-03-31·Journal of Laboratory Medicine and Quality Assurance
Reference Intervals for Research Parameters of Reticulocyte Hemoglobin and Platelet Clumps in Healthy Korean Adults
作者: Kim, Miyoung ; Lee, Jiwon ; Kang, Hee Jung ; Kim, Han-Sung ; Kim, Nan Young ; Jeon, Kibum ; Lee, Young Kyung ; Hong, Sangkyoon
3
项与 Green Cross Laboratories 相关的新闻(医药)2022-11-30
Together with My Green Lab, Agilent is helping support customers in achieving their lab sustainability goals
SANTA CLARA, Calif.--(BUSINESS WIRE)-- Agilent Technologies Inc. (NYSE: A) today announced that it has become the first ‘Angel’ level sponsor of My Green Lab, a nonprofit organization dedicated to building a global culture of sustainability in science.
‘Angel’ level sponsorship provides Agilent access to even more strategic counsel from My Green Lab in support of its sustainability activities and expertise that can be shared with customers.
Agilent is also the proud sponsor of the My Green Lab Certification program – considered the gold standard for laboratory sustainability best practices. As a good citizen and role model for customers, Agilent is working to ensure its on-site customer demonstration laboratories globally are Green Lab Certified and have already achieved highest level certification for Waldbronn, Germany, Cheadle, UK, and Santa Clara, US sites.
“Sustainability and environmental, social, and governance (ESG) standards are central to our mission to advance the quality of life, and our sponsorship of My Green Lab helps us to drive this forward,” said Darlene Solomon, senior vice president, and chief technology officer at Agilent. “Being an ‘Angel’ level sponsor will further cultivate our knowledge of creating sustainable green labs, which we can share with our customers globally and support them on their sustainability journey.”
The Green Lab Certification from My Green Lab is recognized by the United Nations Race to Zero campaign as a key measure of progress for pharmaceutical and medical technology companies toward a zero-carbon future. It indicates that a lab has achieved strong sustainability practices in energy use, water consumption, recycling, and waste production.
The certification builds upon Agilent’s ongoing partnership with My Green Lab, which includes achieving My Green Labs’ ACT (Accountability, Consistency and Transparency) labels which provide consumers with information on the environmental impact of Agilent instruments. Agilent’s spectroscopy division is the latest to receive ACT labels for their Vaya instrument, with more to follow.
About Agilent Technologies
Agilent Technologies Inc. (NYSE: A) is a global leader in the life sciences, diagnostics, and applied chemical markets, delivering insight and innovation that advance the quality of life. Agilent’s full range of solutions includes instruments, software, services, and expertise that provide trusted answers to our customers' most challenging questions. The company generated of $6.85 billion in fiscal 2022 and employs 18,000 worldwide. Information about Agilent is available at . To receive the latest Agilent news, please subscribe to the Agilent Newsroom. Follow Agilent on LinkedIn, Twitter, and Facebook.
2022-06-16
June 16, 2022 13:00 UTC
YONGIN, South Korea--(BUSINESS WIRE)-- GC Labs today announced that GC Labs and its affiliates including GC Cell, GC MS and GC Genome will participate in the IFCC WorldLab 2022 as a platinum sponsor held on 26-30 June 2022 at Coex Convention & Exhibition Center in Seoul.
IFCC WorldLab Congress is the world’s most prestigious academic conference for the lab profession, attended by approximately 4,000 clinical chemistry and laboratory medicine experts from 100 countries to share the latest advances in clinical laboratory science and diagnostics technology.
These four organizations will host a highly interactive booth under the theme of 'Journey to Healthy Life'. Designed to resemble the interior of an airport terminal, the booth will also consist of exciting “experience zones” offering: a virtual reality tour of a GC Labs laboratory using Matterport technology; an opportunity to experience GCare Lipid of GC MS, a personal in-vitro diagnostic product that can measure cholesterol, triglycerides and more, and receive test results.
In addition, they will hold an academic seminar to raise the importance of diagnostic tests on 27-28 June. The seminar will be composed of laboratory medicine evolving after Corona 19 pandemic; Artificial intelligence(AI)-based gene analysis technology; application of ADPS technology liquid biopsy & PCT(Procalcitonin) biopsy method.
“It is significant in that four organizations with their own specialized fields at each stage of the laboratory medicine process gather to create various synergies and introduce total healthcare services.” said Dr. Eun-hee Lee, President of GC Labs. “This congress will be a significant opportunity to share trends and outlooks on global laboratory medicine.”
About GC Labs
GC Labs is the South Korea’s leading clinical laboratory, a specialist in infectious diseases, and serves as part of the Global Diagnostics Network. GC Labs has enabled patients to receive accurate diagnoses and the right treatment with unrivaled quality of routine and specialized clinical tests. Around 800 employees at GC Labs offer more than 5,000 tests and test combinations, ranging from routine tests to highly esoteric molecular and genetic assays. With more than 40 years of accumulated know-how, GC Labs values the principles of providing the best treatment for patients even in unpredictable medical environments through passionate and ceaseless efforts. Not only domestically, but GC Labs has managed to expand overseas by entering a Lab Service Agreement with different areas of the world such as Sri Lanka, Vietnam, Myanmar, Indonesia, India, UAE, Jordan, Cambodia, Bahrain, Saudi Arabia and etc. GC Labs is qualified with excellent medical standard of medical manpower, level, infrastructure, and etc. For further information, please visit our official website
合作
2021-10-25
Improving efficiency and accuracy for microbiology tests
YONGIN, South Korea--(BUSINESS WIRE)-- GC Labs (CEO Eun-hee Lee), the leading clinical diagnostics company in Korea, announced on Oct. 12th that it has introduced WASPLab® (bioMerieux, France), the first microbiology Total Laboratory Automation system in Korea.
When a sample arrives, a clinical microbiological lab receives the sample, and then usually inoculates the sample into a medium and cultures it overnight. After culturing, a tester checks whether the sample is positive. If positive, it moves on to identification and antimicrobial susceptibility tests, and afterwards the results are reported. To date, all of these test steps have been conducted manually, and the type of inoculated medium and the culture time vary depending on the type of sample.
WASPLab®, recently first introduced in Korea by GC Labs, is an automated test system that automatically processes the series of steps from sample receipt to inoculation, culture, and interpretation, which had all been conducted manually. In particular, it automatically interprets the culture as positive or negative and allows test staff to easily double-check images on the monitor before reporting the final results to clinical professionals.
“WASPLab® greatly improves test speed and efficiency, allowing us to report results in just two days for negative urine culture tests and three days for positive urine culture tests, including identification and antimicrobial susceptibility tests, which traditionally took at least four days,” said Ye-jin Oh, Medical doctor of Laboratory medicine department at GC Labs, “It also improves the reproducibility and accuracy of testing and minimizes lost or mistaken samples.”
GC Labs said it will officially begin to use the system in March next year after a 6-month period of evaluation. The company expects to further improve competitiveness in terms of test efficiency and quality with the introduction of the first microbiology total laboratory automation system in Korea.
About GC Labs
GC Labs is the South Korea’s leading clinical laboratory, a specialist in infectious diseases, and serves as part of the Global Diagnostics Network. GC Labs has enabled patients to receive accurate diagnoses and the right treatment with unrivaled quality of routine and specialized clinical tests. Around 800 employees at GC Labs offer more than 5,000 tests and test combinations, ranging from routine tests to highly esoteric molecular and genetic assays. With more than 35 years of accumulated know-how, GC Labs values the principles of providing the best treatment for patients even in unpredictable medical environments through passionate and ceaseless efforts. Not only domestically, but GC Labs has managed to expand overseas by entering a Lab Service Agreement with different areas of the world such as Sri Lanka, Vietnam, Myanmar, Indonesia, India, UAE and etc. GC Labs is qualified with excellent medical standard of medical manpower, level, infrastructure, and etc. For further information, please visit our official website .
合作
100 项与 Green Cross Laboratories 相关的药物交易
登录后查看更多信息
100 项与 Green Cross Laboratories 相关的转化医学
登录后查看更多信息
组织架构
使用我们的机构树数据加速您的研究。
登录
或
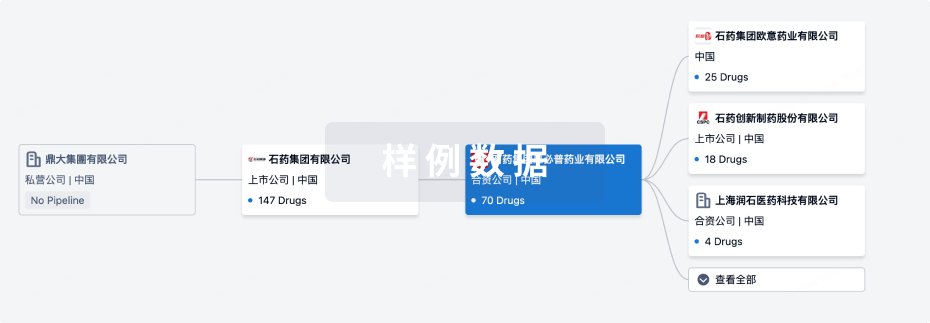
管线布局
2024年12月19日管线快照
无数据报导
登录后保持更新
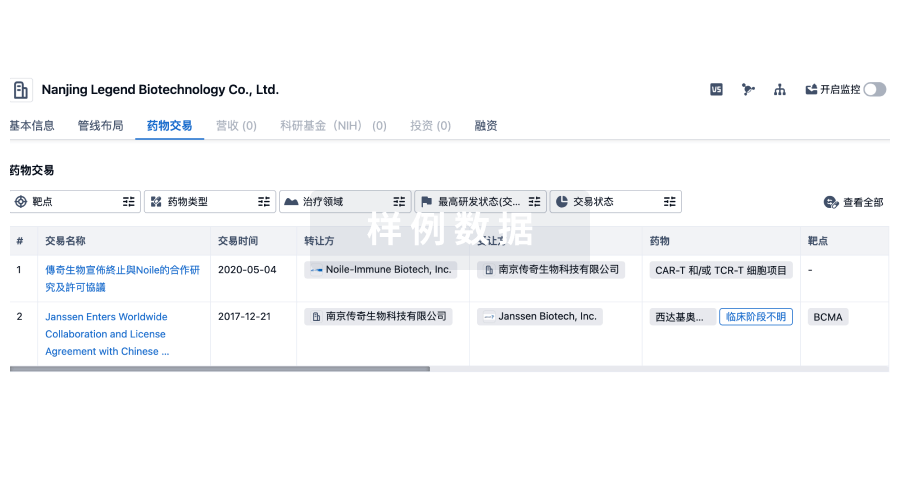
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
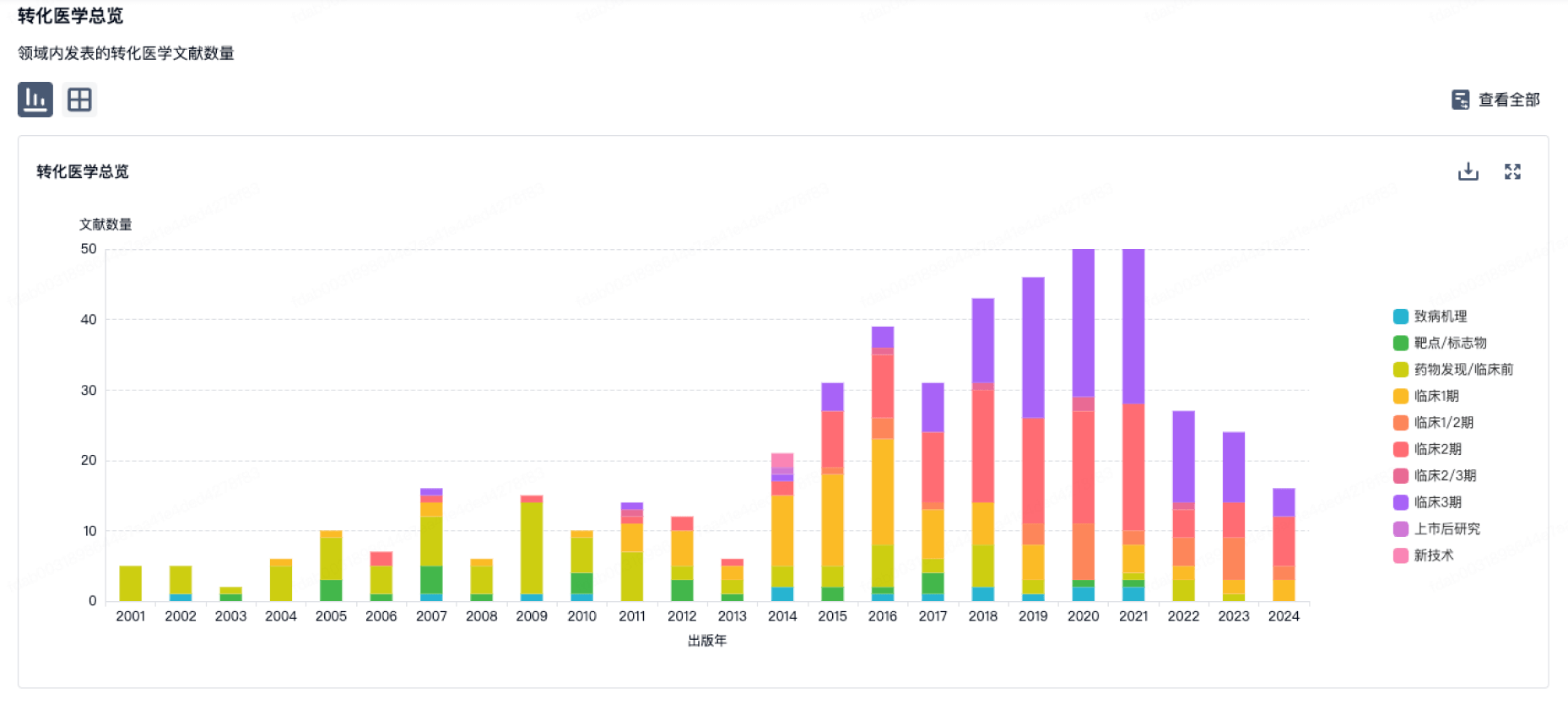
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
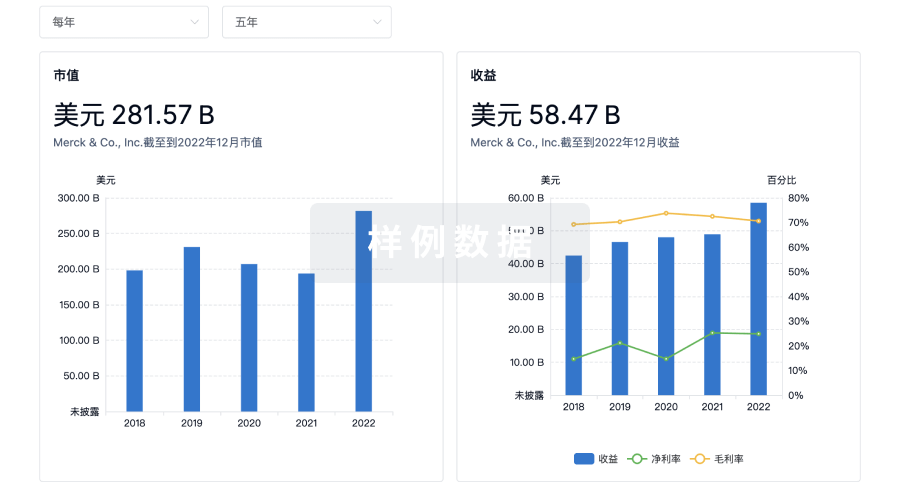
营收
使用 Synapse 探索超过 36 万个组织的财务状况。
登录
或
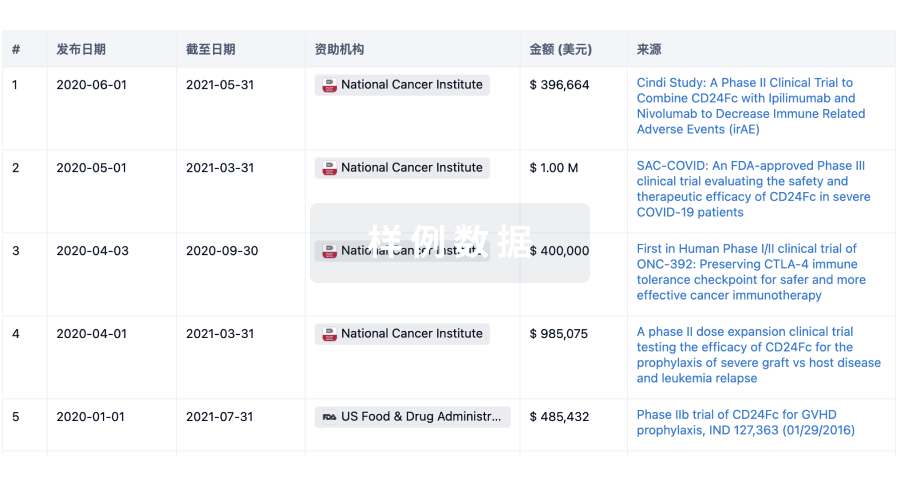
科研基金(NIH)
访问超过 200 万项资助和基金信息,以提升您的研究之旅。
登录
或
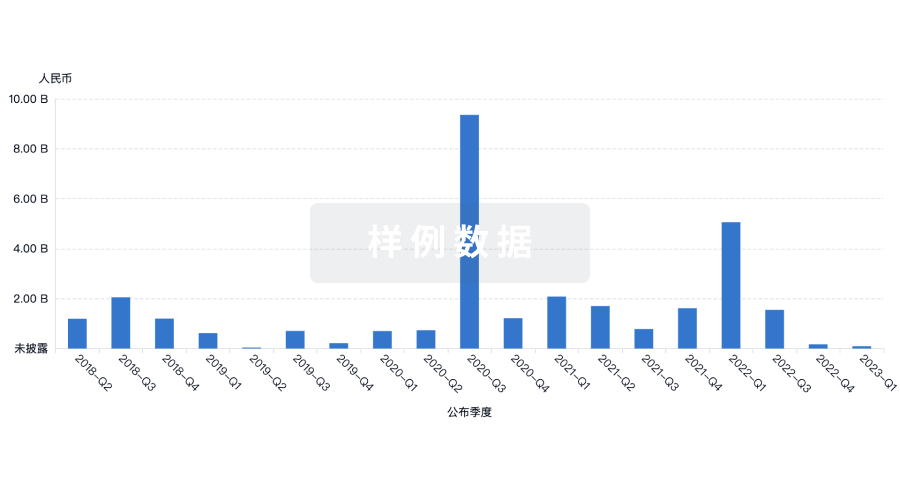
投资
深入了解从初创企业到成熟企业的最新公司投资动态。
登录
或
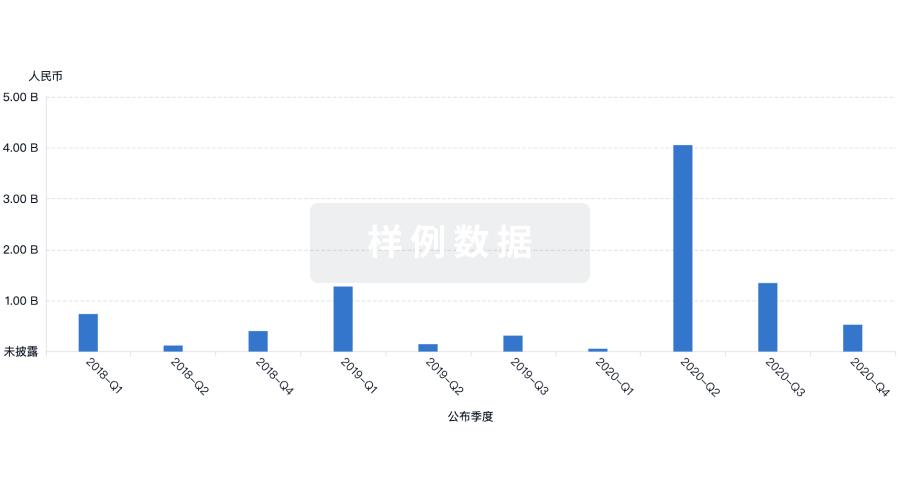
融资
发掘融资趋势以验证和推进您的投资机会。
登录
或
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用