LEVEE MEDICAL™ ANNOUNCES THE CLOSING OF $6.6M SERIES A FINANCING
2022-08-23
Company to Accelerate Development of Novel Urologic Device Designed to
Improve Recovery Outcomes Post-Prostatectomy
DURHAM, N.C., Aug. 23, 2022 /PRNewswire/ -- Levee Medical, a medical device company designing solutions to improve surgical outcomes following prostate cancer, announced today it has closed an initial oversubscribed series A financing round, raising a total of $6.6 million. The funds will be used to advance product development, scale infrastructure, and expand the team to support the development of the company's Voro™ Urologic Scaffold, a bioresorbable post-prostatectomy implant.
Continue Reading
Preview
来源: PRNewswire
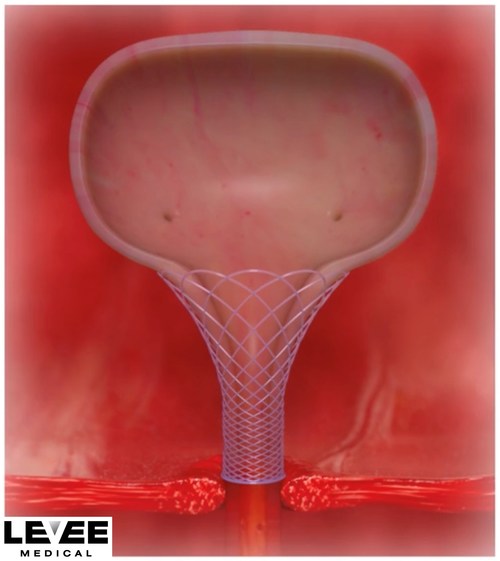
The Voro™ Urologic Scaffold
"We are extremely pleased to secure this financing with strong support from our investors. It reflects their confidence in our ability to address a significant unmet need for patients recovering from radical prostatectomy," says Bruce Choi, Founder, and CTO of Levee Medical. "This positive momentum provides the company with a solid foundation as we move towards achieving important development and clinical milestones."
Prostate cancer is the second most common cancer among men with 1 in 8 diagnosed in their lifetime. The primary type of surgery for prostate cancer is radical prostatectomy. However, there are risks associated with this surgery and nearly all men will experience urinary incontinence following the procedure. This will last a few weeks for many, but up to 15% of patients experience chronic incontinence. Patients are faced with less-than-ideal solutions including pads, catheters, or additional surgery. Current treatments for post-prostatectomy incontinence are invasive, inconvenient, and inadequate.
The Voro Urologic Scaffold is the first and only bioresorbable device designed to be placed during the prostatectomy procedure for the treatment of urinary incontinence. It is designed to reduce the stress on the urinary sphincter by managing the geometry of the bladder neck and maintaining urethral length, which is the best predictor of post-op incontinence.
"I strongly believe the Levee technology could significantly decrease the incidence of post-prostatectomy incontinence and vastly change the way both urologists and patients perceive radical prostatectomy," states Jeffrey Dann, M.D., urologist at the Advanced Urology Institute in Palm Coast, FL, and Former Chairman of AUA New Technology Assessment and Coding and Reimbursement Committees. "Providing patients with a cost-effective solution that allows them to return to daily life without the burden of incontinence represents a much-needed breakthrough in the treatment of prostate cancer."
About Levee Medical
Levee Medical is committed to designing solutions that aim to reduce complications associated with surgical treatment for prostate cancer. The Voro Urologic Scaffold is the first product Levee plans to bring to market. The device is not currently available for sale.
For more information, please visit leveemedical.com
SOURCE Levee Medical
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
靶点
-药物
-来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。



