SURGLASSES Raising $6.5 Million in Pre-A Funding to Accelerate Development and Expansion of AR Augmented Reality Surgical Navigation System - Caduceus S
2023-11-09
TAICHUNG, Nov. 9, 2023 /PRNewswire/ -- SURGLASSES recently raising the successful completion of a $6.5 million Pre-A funding round. This investment was led by Taiwania Capital and saw participation from prominent venture capital firms including Integrated Capital, 500 STARTUPS V, L.P. from the United States, Wisdom Capital Limited Partnership,and Sustainable Impact Capital(SIC), marking a significant milestone in SURGLASSES's growth journey in the medical technology sector. Additionally, it's noteworthy to mention that the initial fundraising target for this round was $5 million, but it was oversubscribed and reached $6.5 million, which demonstrates investors' highly-confidence on SURGLASSES.
Continue Reading
Preview
来源: PRNewswire
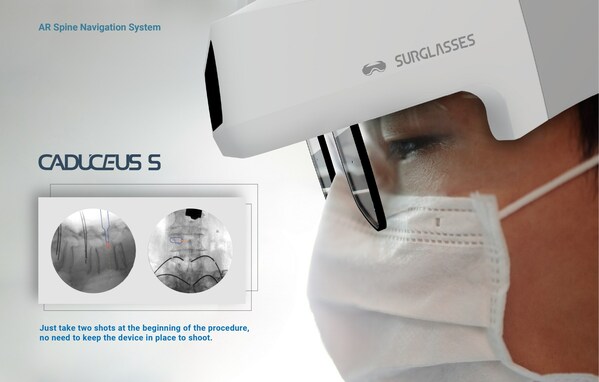
SURGLASSES Caduceus S augmented reality spinal surgery navigation system requires only 2-4 X-rays for navigation.
The funds raised will primarily be directed towards accelerating the research, development, and promotion of SURGLASSES's innovative product - the Caduceus S AR navigation system. Caduceus S is an FDA510(k) Clearance AR medical technology specially designed for complex spinal surgeries. The system allows real-time overlay of 3D spinal anatomy onto the patient during surgical procedures through multiple trackers and a transparent Head-Mounted Display (HMD), providing precise guidance and facilitating surgical operations for surgeons.
In essence, the Caduceus S system projects pre-planned trajectories onto the HMD, offering crucial guidance to surgeons without the need to shift focus to external monitors. This cutting-edge technology ensures a 100% field of view for the surgeon, enabling them to concentrate fully on surgical procedures, ultimately enhancing surgical precision. The seamless integration of the Caduceus S system not only improves surgical accuracy but also enhances ergonomics and efficiency within the operating room.
SURGLASSES believes that the Caduceus S system not only embodies state-of-the-art technology but is also infused with user-centric design, offering greater possibilities for complex spinal surgeries. In the future, SURGLASSES will continue to invest in research and development, contributing further to the advancement of medical technology, ultimately elevating surgical efficiency and accuracy.
Miron Wang, the CEO of SURGLASSES remarked, "Our AR spinal surgery navigation system is currently the most advanced AR system globally. Additionally, we have obtained a patent enabling precise navigation with just one C-arm. Compared to traditional navigation methods, our system exposes patients to less radiation during surgery, ensuring enhanced safety. We are poised to expedite market expansion in the United States."
SURGLASSES extends a warm welcome to professionals from the medtech related field, both domestically and internationally, to join the company and collectively contribute to the research and development of medical technology, propelling the state-of-the-art AR augmented reality surgical navigation system, Caduceus S, to become a benchmark in the global medical community.
For more information, please visit : https://www.surglasses.com. We look forward to future collaboration and communication.
About SURGLASSES
SURGLASSES is a company dedicated to providing AR augmented reality surgical navigation systems for orthopedics and neurosurgery, along with related products, solutions, and services to enhance healthcare intelligence. Our mission is to continually innovate to meet the high-quality and high-efficiency product demands of the medical field while providing advanced tools for medical education.
The company is known for its high-threshold research and development activities, encompassing innovative inventions and software development to address customer needs for medical hardware and software products. We integrate information technology, electronics, and mechanical engineering to create high-quality, world-class products. While our headquarters are based in Taiwan, our goal is to market our products globally.
In 2022, we successfully obtained Taiwan Class II medical device approval for our AR navigation system and achieved FDA 510(k) clearance in the United States by the end of the same year, further validating the high quality and safety of our products. We will continue to work diligently, improving and expanding our product line to better serve our customers, enhance the efficiency of the healthcare industry, and provide high-quality medical education tools.
Media Contact:
Miron Wang, CEO
[email protected]
SOURCE SURGLASSES
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
机构
适应症
-靶点
-药物
-来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。



