Precigen Announces Groundbreaking Pivotal Study Data for PRGN-2012 in Patients with Recurrent Respiratory Papillomatosis in Which More than Half of Patients Achieved Complete Response
2024-06-03
临床结果ASCO会议临床1期细胞疗法加速审批
– Phase 1/2 pivotal study met the primary safety and efficacy endpoints –
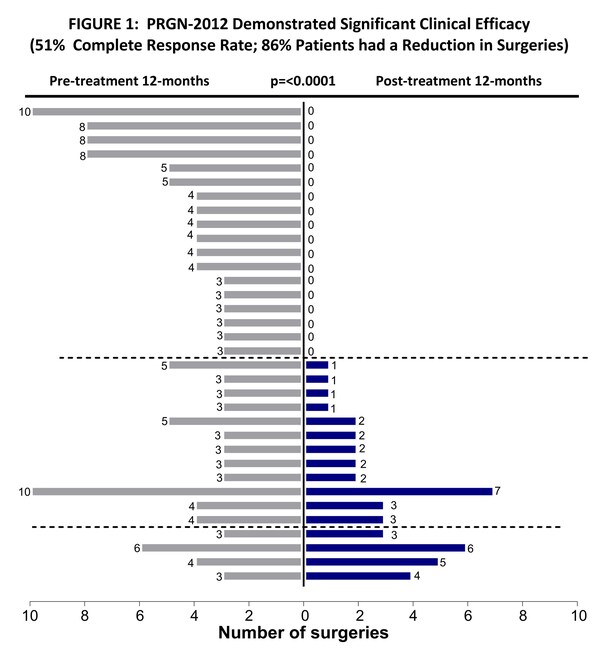
– 51% (18 out of 35) of patients achieved Complete Response, requiring no surgeries after treatment with PRGN-2012; complete responses have been durable beyond 12 months with median duration of follow up of 20 months as of data cutoff –
– 86% of patients (30 out of 35) had a decrease in surgical interventions in the year after PRGN-2012 treatment compared to the year prior to treatment; RRP surgeries reduced from a median of 4 pre-treatment to 0 post-treatment –
– PRGN-2012 was well-tolerated with no dose-limiting toxicities and no treatment-related adverse events greater than Grade 2 –
– PRGN-2012 treatment induced HPV 6/11-specific T cell responses in RRP patients with a significantly greater expansion of peripheral HPV-specific T cells in responders compared with non-responders –
– PRGN-2012 significantly (p
– RRP is a rare, devastating HPV-mediated chronic disease characterized by growth of benign tumors for which the current standard-of-care is repeated surgeries; if approved, PRGN-2012 has the potential to be the first FDA-approved therapeutic for the treatment of RRP –
– Clinical data associated with favorable safety, strong efficacy, ease of administration, and immunological responses, position PRGN-2012 to potentially be the preferred treatment-of-choice for RRP –
– PRGN-2012 rolling BLA submission, under an accelerated approval pathway, is anticipated in the second half of 2024 –
– Precigen to host webcast event today at 6:00 PM CT / 7:00 PM ET –
GERMANTOWN, Md., June 3, 2024 /PRNewswire/ -- Precigen, Inc. (Nasdaq: PGEN), a biopharmaceutical company specializing in the development of innovative gene and cell therapies to improve the lives of patients, today released positive Phase 1/2 pivotal study results for the investigational PRGN-2012 off-the-shelf (OTS) AdenoVerse
® gene therapy in patients with recurrent respiratory papillomatosis (RRP). Results were presented in a late-breaking oral presentation at the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting by Scott M. Norberg, DO, Associate Research Physician, Center for Immuno-Oncology, Center for Cancer ResearchCancer Research, National Cancer InstituteCancer Institute and a lead investigator for the PRGN-2012 clinical study. The Company will host a webcast event today at 6:00 PM CT / 7:00 PM ET to detail the results presented at ASCO.
Continue Reading
Preview
来源: PRNewswire
"We are thrilled with the results of the Phase 1/2 pivotal study showing more than half of patients were surgery free–Complete Response–and 86% of patients had a significant reduction in the need for surgeries after PRGN-2012 treatment. Based on the efficacy, safety, and ease of administration, we believe PRGN-2012 is a game-changer for RRP patients and has the potential to be the preferred treatment-of-choice for RRP," said Helen Sabzevari, PhD, President and CEO of Precigen. "We look forward to sharing these results with the FDA as part of a rolling Biologics License Application submission under an accelerated approval pathway. We have ramped up our commercial readiness efforts in anticipation of a potential launch in 2025 and are excited by the potential to bring a long overdue new treatment option to the RRP community."
Pivotal Study Design and Endpoints
The Phase 1/2 clinical study (clinical trial identifier: NCT04724980) evaluated safety and efficacy of PRGN-2012. The study design included an initial 3+3 dose escalation cohort to identify the recommended Phase 2 dose (RP2D). Adult RRP patients who had three or more surgeries in the prior 12 months were eligible for the study. The Phase 1/2 study enrolled a total of 38 patients. Of these, 3 patients received four administrations of PRGN-2012 at 1x 1011 particle units (PU)/dose and 35 patients received four administrations of PRGN-2012 at RP2D (5 x 1011 PU/dose) over a 12 week treatment period via subcutaneous injection.
Primary endpoints included safety and Complete Response rate defined as the percentage of patients who require no RRP surgeries in the 12-month period after PRGN-2012 treatment completion. Key secondary endpoints included HPV-specific immune responses, extent of papilloma growth as measured by Derkay scoring, and quality of life measurement as measured by Vocal Handicap Index-10 (VHI-10).
Patient Characteristics
Baseline patient characteristics of the 35 adult patients included a median age of 49 years (range: 20-88); 20 of the patients were male and 15 were female. Patients had a median of 4 surgeries (range: 3-10) in the 12 months before PRGN-2012 treatment initiation. Average years since RRP diagnosis was 20 (range: 1-65) with 12 and 23 patients with juvenile and adult onset RRP, respectively.
Clinical Efficacy
Primary efficacy endpoint analysis demonstrated that 51% (18 out of 35) (95% CI: 34-69) patients achieved Complete Response, defined as no need for RRP surgeries in the 12-month period following completion of PRGN-2012 treatment. The Complete Response rate was 50% (6 out of 12) and 52% (12 out of 23) in the Phase 1 and Phase 2 portions of the study, respectively (TABLE 1). Complete Responses were durable. Median durability of response has not yet been reached with median follow up of 20 months as of the data cutoff date of May 20, 2024. PRGN-2012 treatment significantly (p .
PRGN-2012 treatment showed significant (p
Safety
PRGN-2012 treatment was well-tolerated with no dose-limiting toxicities and no treatment-related adverse events (TRAEs) greater than Grade 2 (TABLE 2). All patients received four administrations of PRGN-2012 at the intended dose levels. TRAEs were mostly mild with no treatment-related serious adverse events reported. The most common TRAE was injection site reaction. Other common TRAEs occurring in more than one subject were fatigue, chills, and fever. There was no meaningful anti-drug antibody response with repeat administrations of PRGN-2012.
SOURCE Precigen, Inc.
更多内容,请访问原始网站
文中所述内容并不反映新药情报库及其所属公司任何意见及观点,如有版权侵扰或错误之处,请及时联系我们,我们会在24小时内配合处理。
适应症
靶点
-来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。





