1
项与 Ad26.CS.01(Crucell Holland BV) 相关的临床试验A Phase I/IIa, Double-blind, Randomized, Placebo-controlled, Dose-escalation Clinical Study Evaluating Safety, Tolerability and Immunogenicity of Two Dose Levels of Recombinant Adenoviral Serotype Ad35 and Serotype Ad26 Vectors Expressing the Malaria Plasmodium Falciparum Circumsporozoite Antigen Administered as Heterologous Prime-boost Regimen, and Assessing Protective Efficacy of the Higher Dose in a Malaria Challenge Model in Unblinded Conditions
The purpose of this study is to assess the safety, tolerability and immunogenicity of two dose levels (1x10^10 and 5x10^10 virus particles (vp)) of the adenovirus serotype (Ad) Ad35.CS.01/Ad26.CS.01 prime-boost malaria candidate vaccine, followed by an evaluation of the protective efficacy of the higher dose level in an experimental malaria challenge.
The study will be in 3 phases:
a dose escalation / vaccination phase in which both dose levels will be tested
a malaria challenge phase in which only subjects receiving the Ad35.CS.01/Ad26.CS.01 5x10^10 vp dose level, together with six infectivity control subjects, will be exposed to experimental challenge with Plasmodium falciparum
a long term follow up phase in which all subjects who received active vaccine from both dose levels and/or malaria challenge will be included
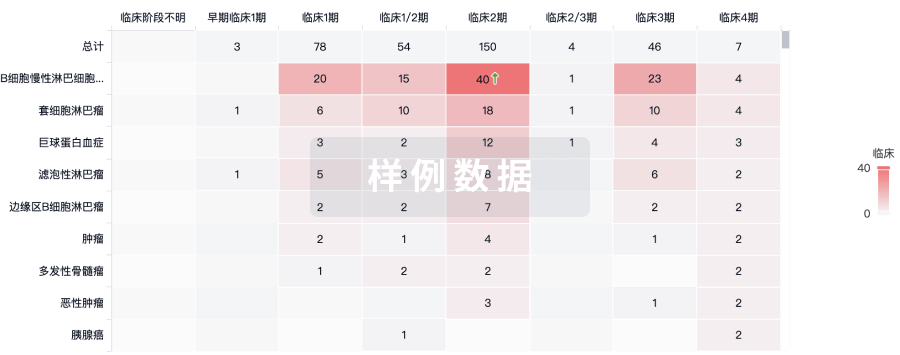
100 项与 Ad26.CS.01(Crucell Holland BV) 相关的临床结果
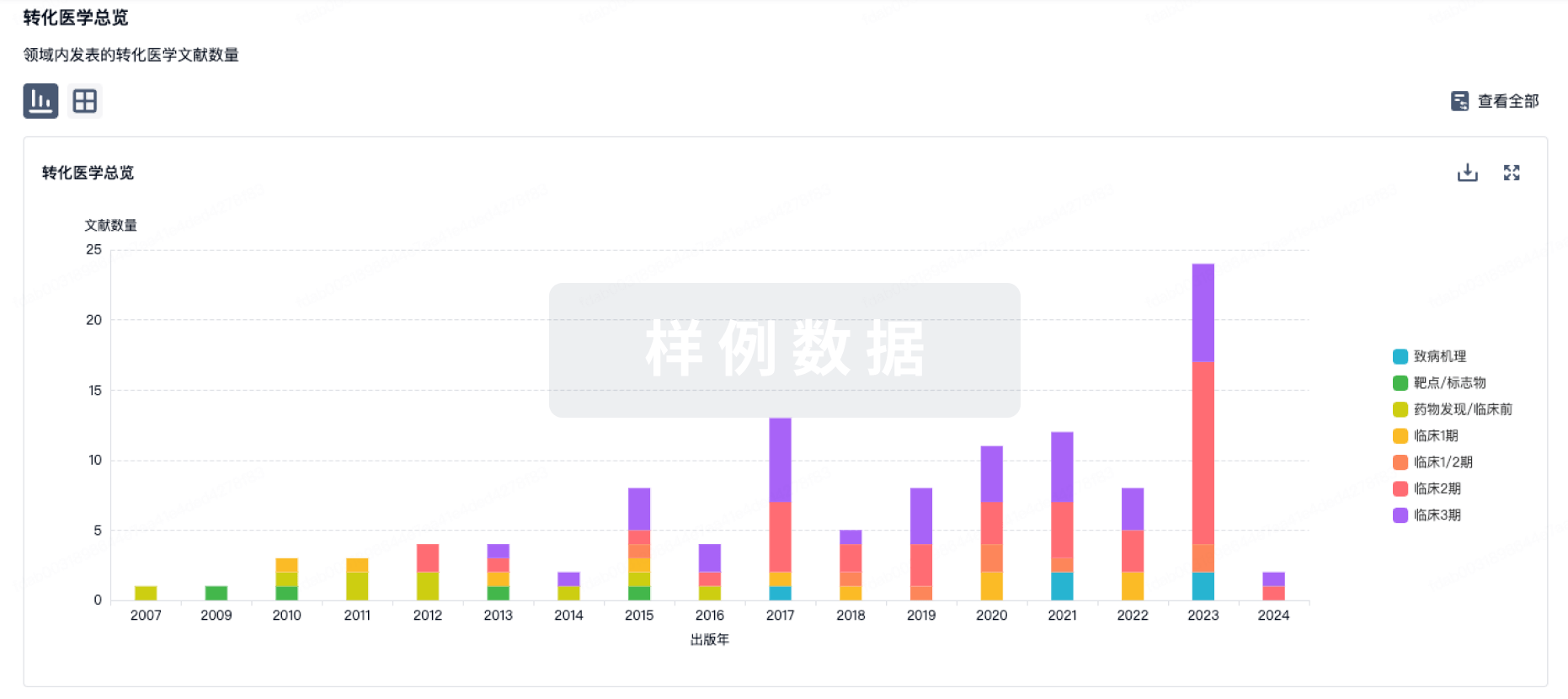
100 项与 Ad26.CS.01(Crucell Holland BV) 相关的转化医学
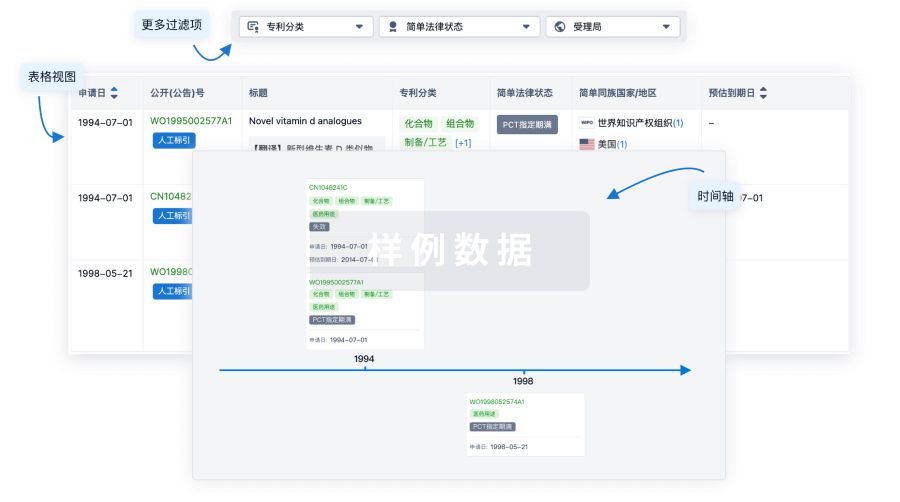
100 项与 Ad26.CS.01(Crucell Holland BV) 相关的专利(医药)
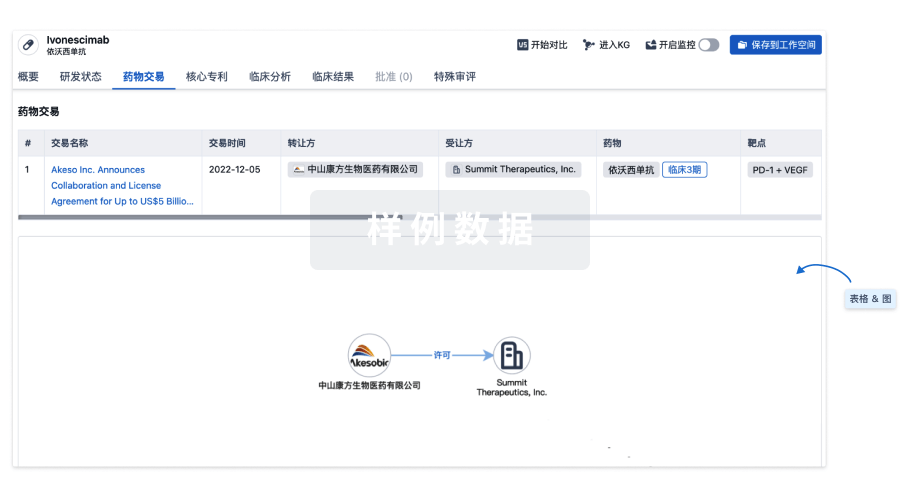
100 项与 Ad26.CS.01(Crucell Holland BV) 相关的药物交易