预约演示
更新于:2025-03-20
SPI-40
更新于:2025-03-20
概要
基本信息
在研机构- |
最高研发阶段无进展药物发现 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
关联
100 项与 SPI-40 相关的临床结果
登录后查看更多信息
100 项与 SPI-40 相关的转化医学
登录后查看更多信息
100 项与 SPI-40 相关的专利(医药)
登录后查看更多信息
82
项与 SPI-40 相关的文献(医药)2024-08-01·Investigational New Drugs
Radiosensitization effect of quinoline-indole-schiff base derivative 10E on non-small cell lung cancer cells in vitro and in tumor xenografts
Article
作者: Liu, Duanya ; Liu, Hongwei ; Lan, Wanying ; Yao, Jie ; Wang, Qianqian ; Huang, Jiangang
2024-08-01·The Journal of Pharmacology and Experimental Therapeutics
Preclinical Systemic Pharmacokinetics, Dose Proportionality, and Central Nervous System Distribution of the ATM Inhibitor WSD0628, a Novel Radiosensitizer for the Treatment of Brain Tumors
Article
作者: Zhong, Wei ; Zhang, Wenjuan ; Le, Jiayan ; Oh, Ju-Hee ; Garcia, Darwin A ; Mladek, Ann C ; Sarkaria, Jann N ; Xue, Zhiyi ; Zhang, Wenqiu ; Elmquist, William F ; Burgenske, Danielle M ; Rathi, Sneha
2023-12-01·Cancer Nanotechnology
Dual enhancement in the radiosensitivity of prostate cancer through nanoparticles and chemotherapeutics
Article
作者: Chithrani, Devika Basnagge ; Abousaida, Belal ; Mackeyev, Yuri ; Krishnan, Sunil ; Beckham, Wayne ; Morgan, Jessica ; Jackson, Nolan ; Herchko, Steven ; Bromma, Kyle ; Alhussan, Abdulaziz ; Hill, Iona ; Zahra, Yasmin
100 项与 SPI-40 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 肿瘤 | 药物发现 | 美国 | - |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
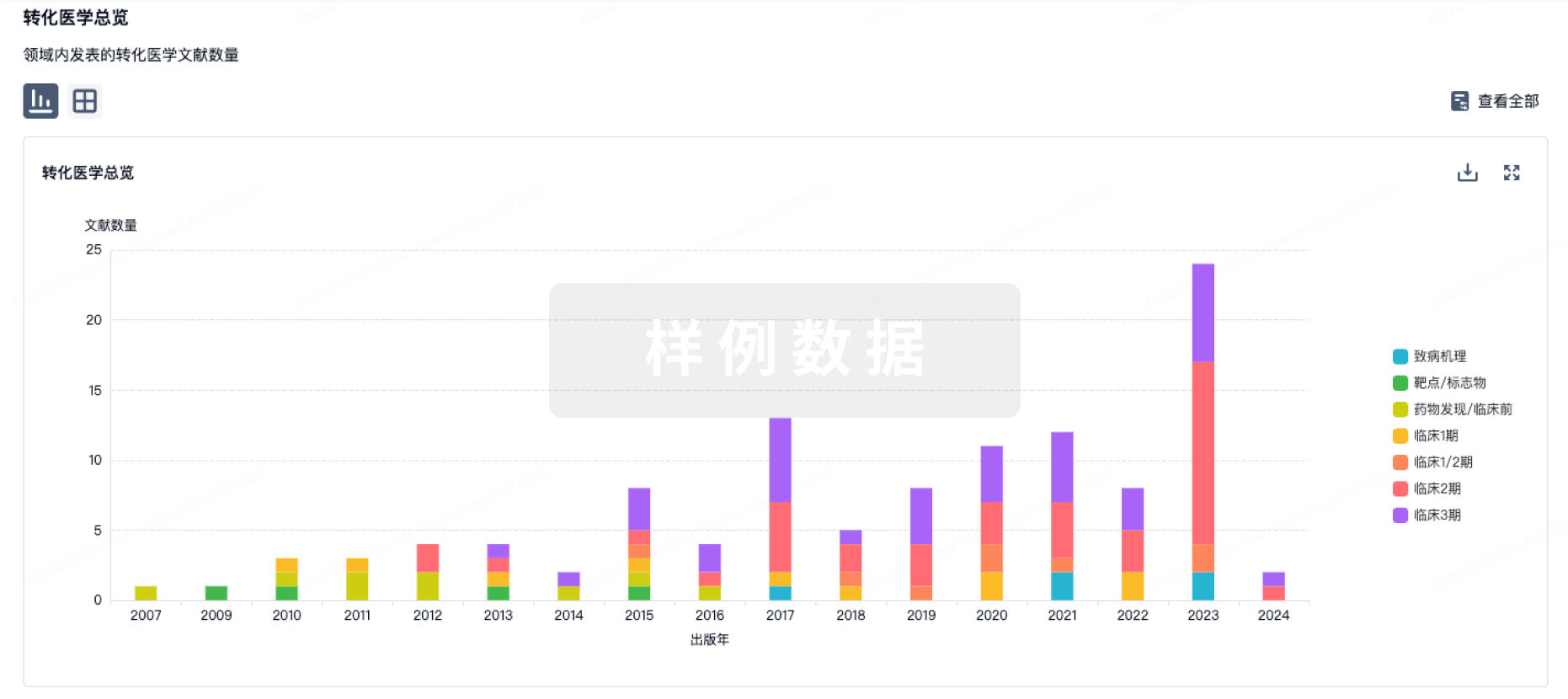
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
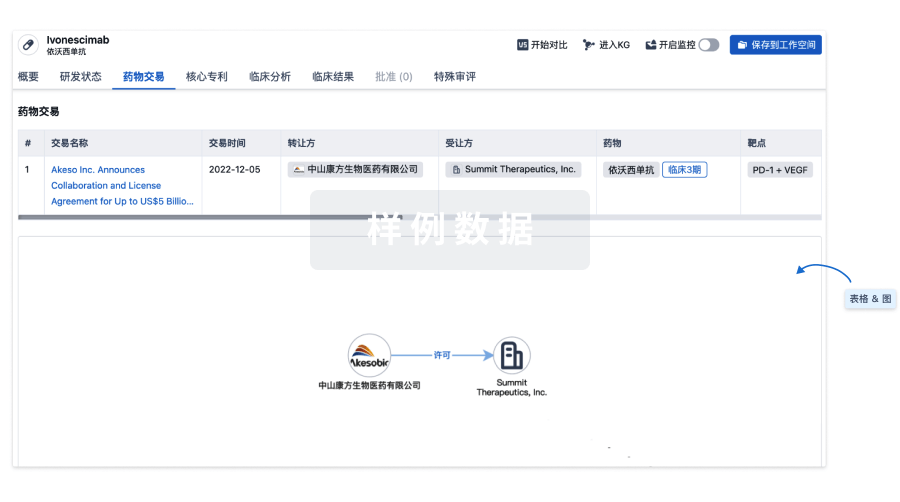
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
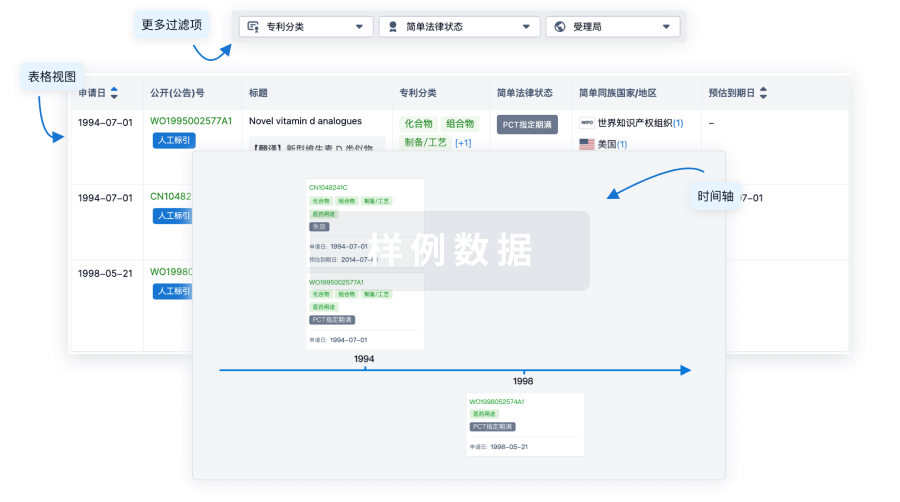
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
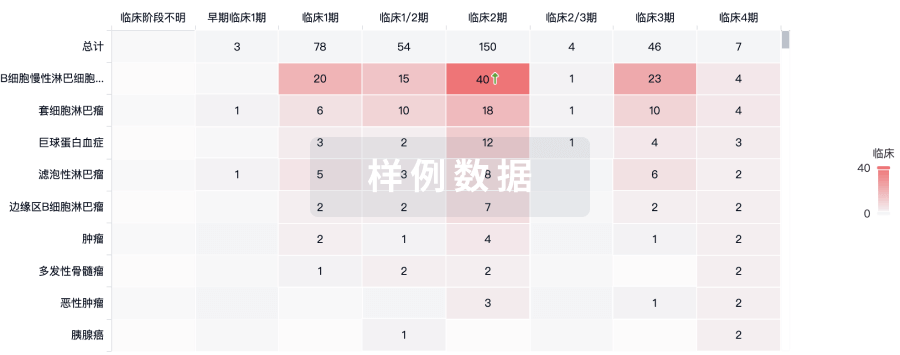
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用