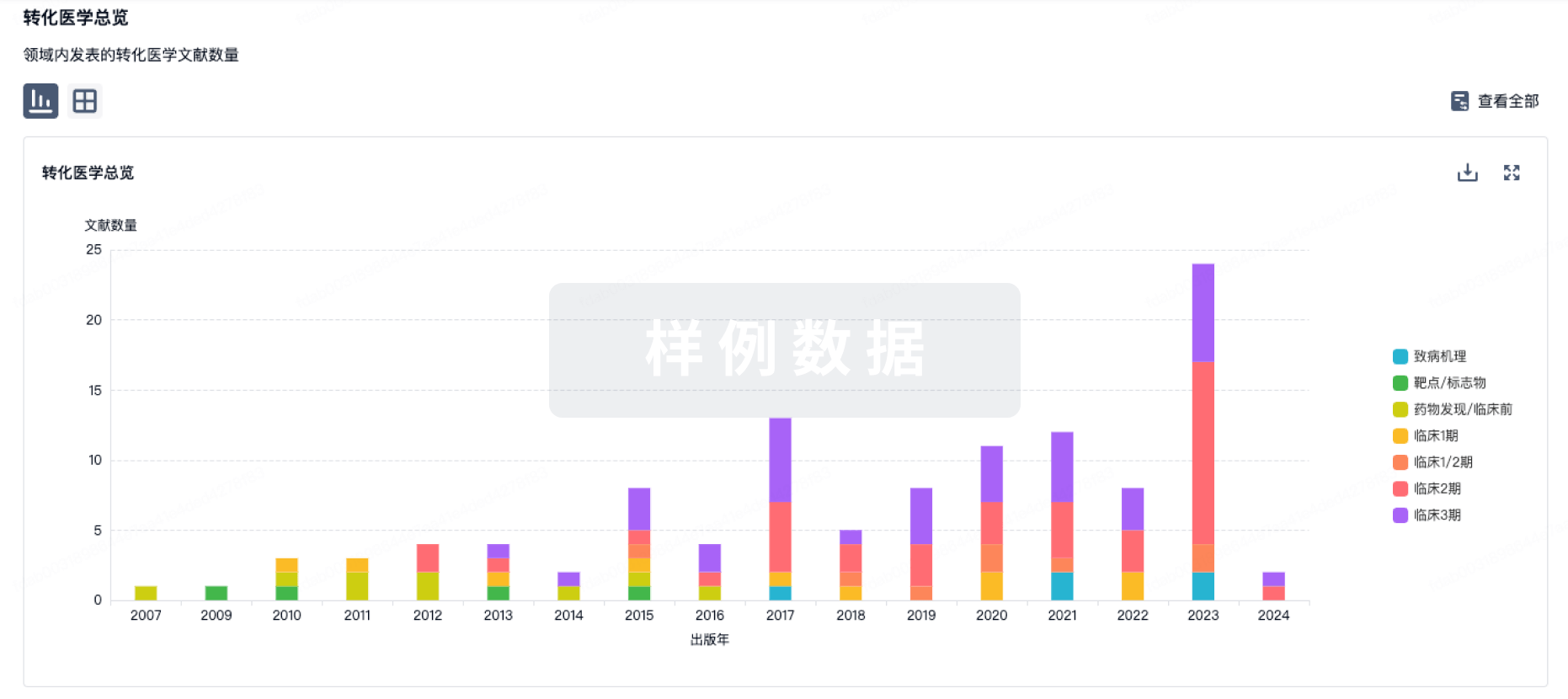
WATERTOWN, Mass., November 5, 2024 – Orna Therapeutics, a biotechnology company dedicated to designing and delivering a new class of circular RNA medicines and unprecedented lipid nanoparticle (LNP) delivery solutions for oncology and autoimmune diseases, today announced an upcoming poster presentation at the 66th American Society of Hematology (ASH) Annual Meeting in San Diego from December 7-10, 2024. The presentation will outline data supporting the exploration of its in vivo CAR therapy approach in oncology and autoimmune diseases. Presentation details are as follows:
Title: In Vivo panCAR-Mediated Depletion of B cells in Non-Human Primates Using a Circular (oRNA®) Anti-CD20 CAR
Speaker: Dr. Akinola Emmanuel, Principal Scientist, Drug Discovery, Orna Therapeutics
Date/Time: Sunday, December 8, 20246:00 PM – 8:00 PMPT
Publication Number: 3427
Session Name: 702. CAR-T Cell Therapies: Basic and Translational: Poster II
About Orna Therapeutics
Orna Therapeutics is dedicated to designing and delivering a new class of fully engineered circular RNA (oRNA®) therapeutics to unlock the potential of RNA medicine to treat diseases anywhere in the body. Orna’s circular RNA transcripts have advantages over traditional mRNA approaches, including simplified production, improved formulation into lipid nanoparticles, and superior protein expression. Its industry-leading LNP-based delivery systems and comprehensive editing programs position Orna to advance novel RNA medicines with vast potential to transform patient care. To learn more, visit: www.ornatx.com and follow Orna Therapeutics on Twitter and LinkedIn.
Investor Contact:
Investor-relations@ornatx.com
Media Contact:
Peg Rusconi
Deerfield Group
Peg.rusconi@deerfieldgroup.com