预约演示
更新于:2025-01-23
Antihemophilic Factor (Human) (CSL Behring)
更新于:2025-01-23
概要
基本信息
原研机构 |
在研机构 |
非在研机构- |
最高研发阶段批准上市 |
首次获批日期 美国 (2003-05-14), |
最高研发阶段(中国)- |
特殊审评- |
登录后查看时间轴
关联
1
项与 Antihemophilic Factor (Human) (CSL Behring) 相关的临床试验NCT01051544
Randomised Study of First TIME Immunotolerance Induction in Patients With Severe Type A Haemophilia With Inhibitor at High Risk of Failure: Comparison of Induction of Immune Tolerance With FVIII Concentrates With or Without Von Willebrand Factor Acronym: RES.I.S.T.- Naive
This is a prospective, controlled, randomized, open label study, aimed at comparing FVIII/VWF concentrates with FVIII concentrates at 200 IU/kg daily in their ability to induce immune tolerance in Haemophilia A patients with high responding inhibitors and poor prognosis for success.
开始日期2009-09-25 |
申办/合作机构 |
100 项与 Antihemophilic Factor (Human) (CSL Behring) 相关的临床结果
登录后查看更多信息
100 项与 Antihemophilic Factor (Human) (CSL Behring) 相关的转化医学
登录后查看更多信息
100 项与 Antihemophilic Factor (Human) (CSL Behring) 相关的专利(医药)
登录后查看更多信息
20
项与 Antihemophilic Factor (Human) (CSL Behring) 相关的文献(医药)2003-09-01·Haemophilia2区 · 医学
Impact of inhibitor epitope profile on the neutralizing effect against plasma‐derived and recombinant factor VIII concentrates in vitro
2区 · 医学
Article
作者: Erik Berntorp ; A. Shapiro ; Jan Astermark ; G. Tjönnfjord ; D. DiMichele ; J. Voorberg ; H. Lenk
2001-03-14·Haemophilia2区 · 医学
Viral safety of a pasteurized, monoclonal antibody‐purified factor VIII concentrate in previously untreated haemophilia A patients
2区 · 医学
Article
作者: Philipp, C S
2000-03-01·Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis
Pasteurized, monoclonal antibody factor VIII concentrate: establishing a new standard for purity and viral safety of plasma-derived concentrates.
Review
作者: Goldsmith, J C
100 项与 Antihemophilic Factor (Human) (CSL Behring) 相关的药物交易
登录后查看更多信息
研发状态
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 血友病A | 美国 | 2003-05-14 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
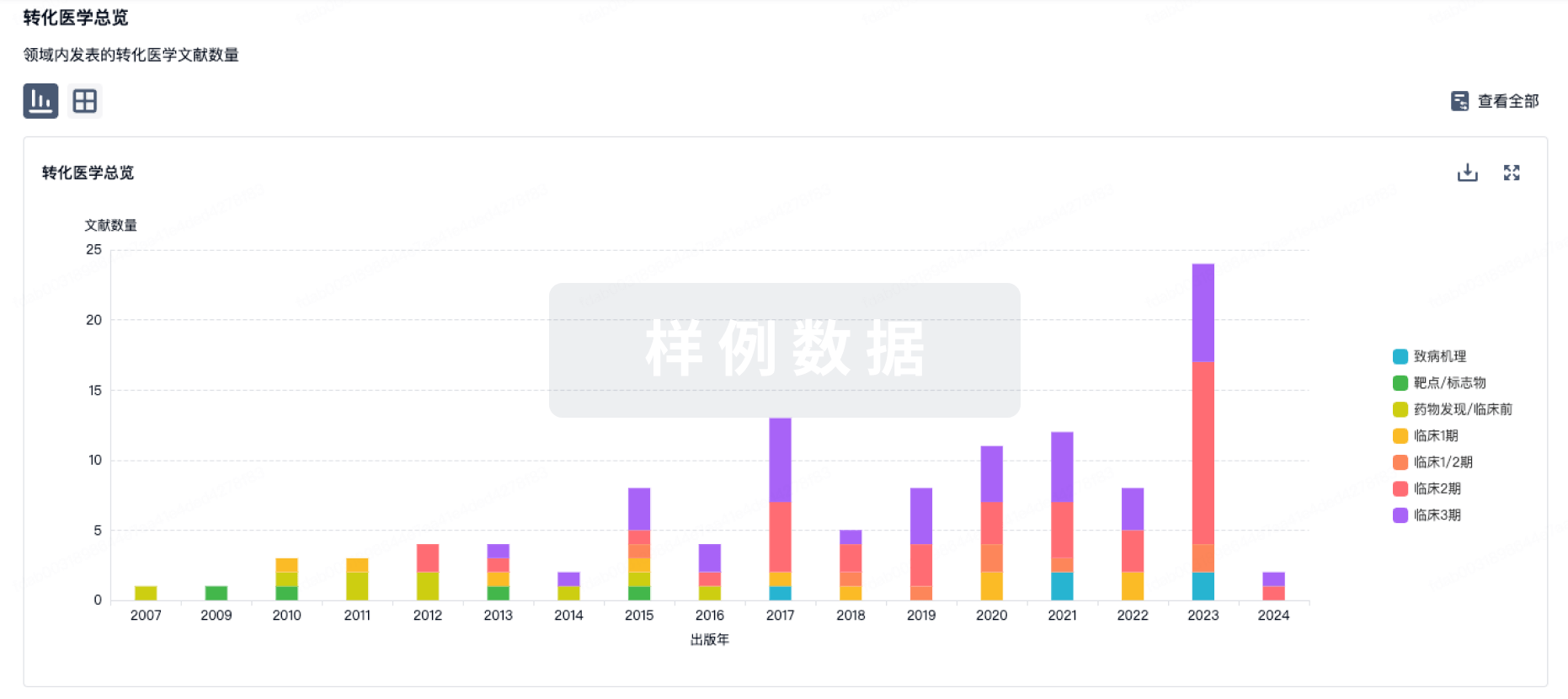
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
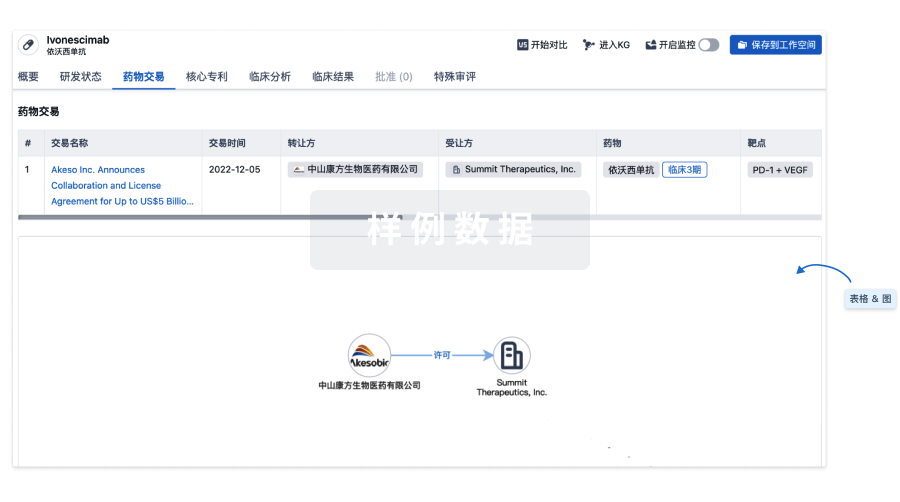
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
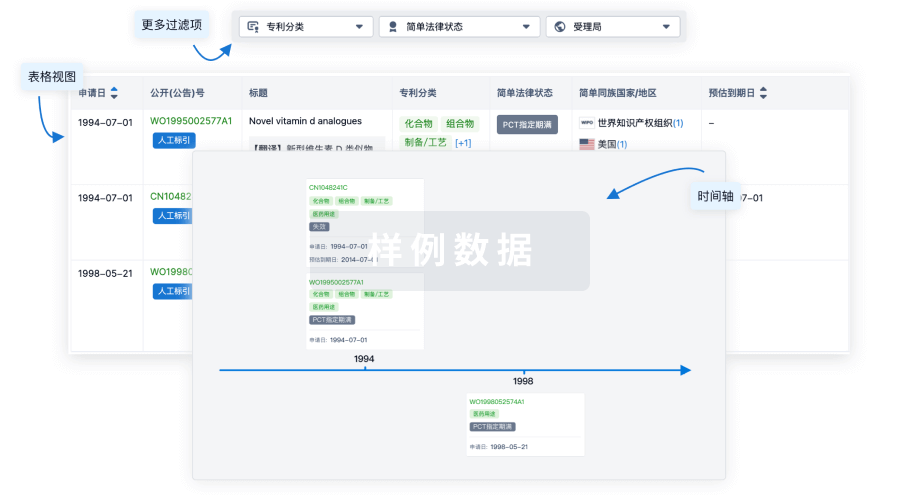
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
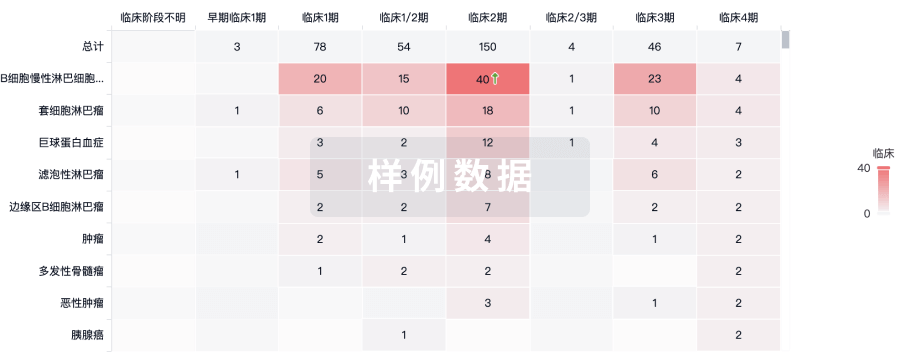
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

