预约演示
更新于:2025-06-28
SRSD-101
更新于:2025-06-28
概要
基本信息
原研机构 |
在研机构 |
非在研机构- |
权益机构- |
最高研发阶段临床1期 |
首次获批日期- |
最高研发阶段(中国)临床1期 |
特殊审评- |
登录后查看时间轴
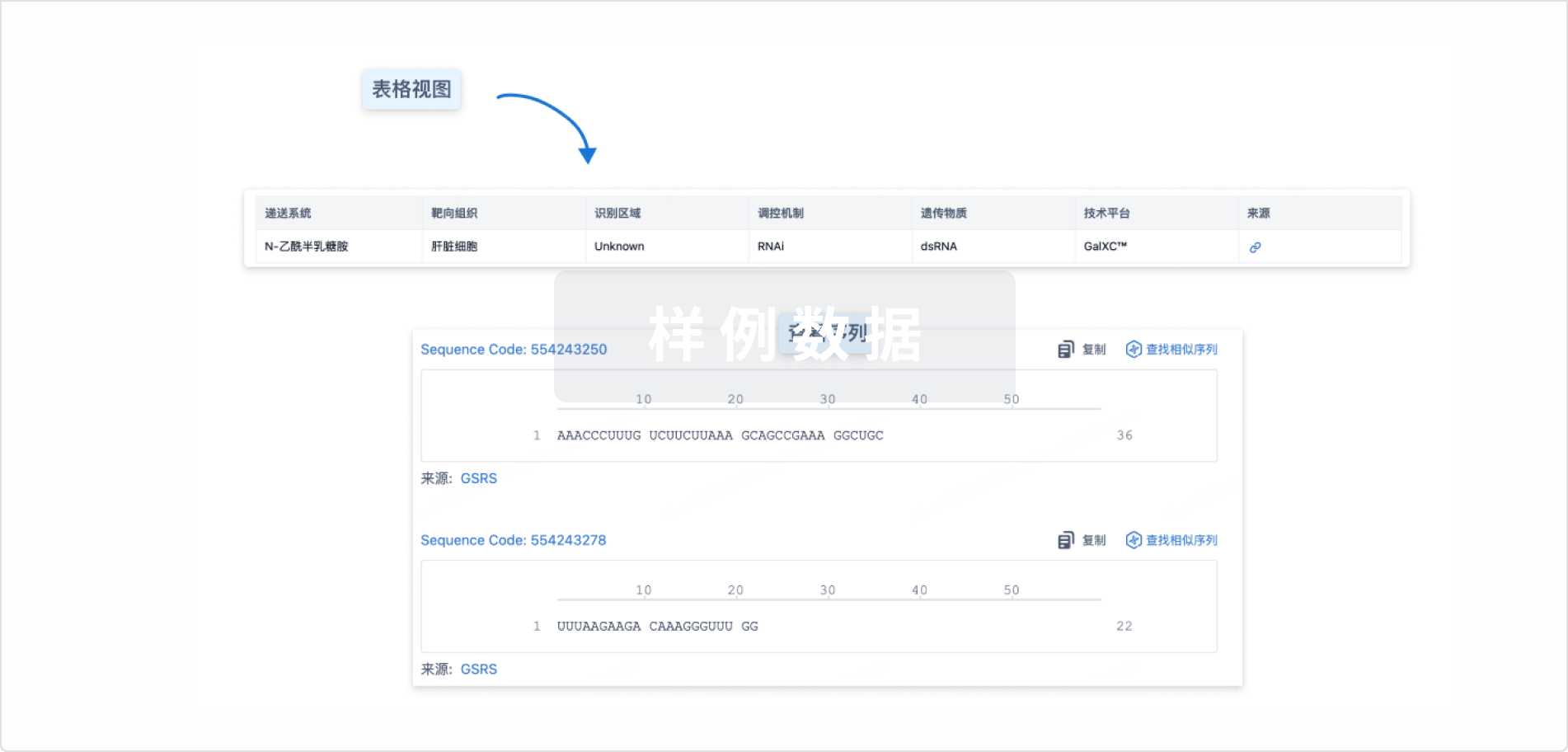
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
关联
1
项与 SRSD-101 相关的临床试验CTR20233758
一项在低密度脂蛋白胆固醇正常或升高的中国受试者中评价SRSD101 注射液单剂量皮下给药剂量递增安全性、耐受性、药代动力学和药效学特征的随机、双盲、安慰剂对照的I期临床研究
主要目的:评价单剂量皮下给药后SRSD101 在LDL C 正常或升高的中国受试者中的安全性和耐受性。
次要目的:评价单剂量皮下给药后SRSD101 及其血浆代谢产物(如适用)在LDL C 正常或升高的中国受试者中的药代动力学(PKPK)特征;评价单剂量皮下给药后SRSD101 在LDL C 正常或升高的中国受试者中的药效学(PDPD)特征;评价单剂量皮下给药后SRSD101 在LDL C 正常或升高的中国受试者中的免疫原性(ADAADA)特征。
开始日期- |
申办/合作机构 |
100 项与 SRSD-101 相关的临床结果
登录后查看更多信息
100 项与 SRSD-101 相关的转化医学
登录后查看更多信息
100 项与 SRSD-101 相关的专利(医药)
登录后查看更多信息
27
项与 SRSD-101 相关的新闻(医药)2025-05-21
·医药笔记
-该合作将双方在研发与产业化方面的互补能力相结合,共同开发并商业化下一代长效Factor XI(FXI)靶向小干扰RNA(siRNA)疗法SRSD107,用于治疗血栓及血栓栓塞性疾病 --根据协议条款,靖因药业将获得CRISPR Therapeutics(纳斯达克股票代码:CRSP)支付的9500万美元现金及现金等价物作为首付款,并有资格获得超8亿美元的预付款和里程碑付款。双方将以50-50的成本和利润分摊机制共同开发SRSD107。此外,协议还授予CRISPR Therapeutics在未来独家引进最多两个额外siRNA项目的优先授权权利 -NEWS美国圣地亚哥、中国上海、瑞士楚格及美国波士顿,2025年5月20日——靖因药业是一家处于临床阶段、专注于开发面向全球市场创新小干扰RNA(siRNA)疗法的生物技术公司;CRISPR Therapeutics(纳斯达克股票代码:CRSP)是一家专注于为严重疾病开发变革性基因疗法的生物制药公司。今日,双方宣布达成战略合作伙伴关系,将携手推进siRNA疗法的联合开发与商业化进程。靖因药业执行董事兼首席执行官冀群升博士“我们非常高兴与CRISPR达成此次合作。CRISPR Therapeutics是基因疗法开发领域公认的全球领导者。血栓性疾病存在大量未被满足的医疗需求,SRSD107展示的令人鼓舞的1期临床数据,进一步验证了其作为靶向凝血因子XI(FXI)同类最佳疗法的潜力。靖因药业致力于为全球患者提供创新治疗方案。此次合作将充分发挥双方优势,携手加速新一代siRNA疗法的全球开发与临床转化。”CRISPR Therapeutics董事长兼首席执行官Samarth Kulkarni博士“我们很高兴与靖因药业建立合作关系,并在近期公布的CTX310项目(靶向ANGPTL3)令人振奋的顶线数据基础上,进一步拓展我们在心血管疾病领域的管线布局。凝血因子XI是一个创新且极具吸引力的靶点,在治疗影响全球数以百万计患者的血栓性疾病方面展现出巨大潜力。SRSD107有望成为同类最佳疗法,凭借更低的给药频率和更优的临床获益,为临床治疗带来重大突破。靖因药业在siRNA平台方面的前沿技术与我们现有的研发能力高度互补,进一步丰富了我们的治疗技术平台,助力我们开发更广泛的变革性基因疗法。”TIMI研究组资深研究员、布里格姆妇科医院心脏病科主任、哈佛医学院副教授Christian T. Ruff医学博士“由于心血管疾病、恶性肿瘤、和高凝状态等基础疾病的存在,大量患者面临潜在的致死性血栓栓塞性事件风险。其中相当一部分患者由于担忧出血风险或用药依从性问题,未能获得治疗或充分治疗。SRSD107有望成为一款具有差异化优势的疗法,其特点包括出血风险更低、给药频率更低,患者依从性更高、无肾脏清除或药物相互作用担忧、并且具备更佳的可逆性,从而进一步降低出血风险的可能性。这些优势使其有望在现有疗法及靶向FXI其它疗法中脱颖而出。”SRSD107是一款新一代的长效siRNA疗法,旨在选择性抑制凝血因子XI(FXI)。FXI靶点在病理性血栓形成中起关键作用,但对正常止血功能的影响较小。通过靶向FXI,SRSD107有望在降低血栓事件发生的同时,显著减少出血风险,展现出有别与凝血因子X活性(FXa)抑制剂的治疗优势。SRSD107的潜在适应症广泛,包括房颤、静脉血栓栓塞症(VTE)、肿瘤相关性血栓、接受血液透析的终末期肾病患者,以及因出血风险限制现有治疗手段的重大骨科手术人群。目前,SRSD107已完成两项1期临床试验,结果显示单次给药安全性且耐受性良好。相关研究成果已在2025年美国心脏病学会(ACC)年会和2024年美国血液学会(ASH)年会上发布。SRSD107的2期临床试验正在启动,旨在评估其在膝关节置换术患者中预防静脉血栓栓塞症(VTE)的安全性和有效性。该研究将为确认其抗凝临床获益及后续关键性试验的剂量选择提供临床科学依据。双方将以50:50的成本与利润分成模式,共同推进SRSD107的开发。根据协议,CRISPR将负责该产品在美国的商业化,靖因药业则负责大中华区市场。此外,CRISPR Therapeutics拥有提名最多两个siRNA靶点的权利,并可选择主导后续的临床开发与商业化。靖因药业将有资格获得与项目进展挂钩的里程碑付款、附带条件的激励付款,以及按销售额分级计算的版税收入。关于血管栓塞血栓形成是血管内限制血液流动的血凝块,可发生在动脉或静脉循环中,是大多数心肌梗死、缺血性脑卒中和静脉血栓栓塞症(VTE)的共同病理基础。全球有四分之一的人死于血栓栓塞引发的疾病1。关于SRSD107注射液SRSD107注射液是一款靖因药业拥有自主知识产权的双链小干扰核酸(siRNA)药物。通过特异性肝靶向人凝血因子XI(FXI )mRNA,抑制FXI的蛋白表达,阻断内源性凝血途径的激活,从而达到抗凝血作用。临床前试验数据显示,单次皮下注射SRSD107,可降低外周血FXI浓度近100%,且持续时间可长达半年,同时未见出血。SRSD107兼具强效持久作用和良好的安全性,有望成为潜在的同类首创(First-in Class)和同类最佳(Best-in-Class)新一代更安全的抗凝药物。关于靖因药业靖因药业 (Sirius Therapeutics) 是一家临床阶段的生物技术公司,以人类健康福祉为使命,致力开发颠覆慢病防治的siRNA新疗法的领军者。目前已进入临床开发阶段的产品包括用于治疗血栓栓塞性疾病的SRSD107,高脂蛋白(a)的SRSD216,和血脂异常的SRSD101。成立于2021年,由国际卓越的管理团队和全球知名医疗健康投资机构孵化。公司采取国际化的战略定位,汇聚中美两地在小核酸疗法领域的人才和资源的优势,建立了以美国为源头创新发现中心,中国为全球转化医学中心的布局。利用其小核酸药物研发核心技术平台,开发了多个具有同类首创或同类最佳潜质的差异化全球竞争优势的产品管线。公司已累计融资近1.5亿美元。欲了解更多信息,请访问www.siriusrna.com.关于CRISPR Therapeutics自十多年前成立以来,CRISPR Therapeutics已从一家致力于推进基因编辑计划的研究型公司发展成为一家行业领导者,并庆祝了全球首个CRISPR基因疗法获批的历史性时刻。该公司拥有多元化的产品候选组合,涵盖广泛的疾病领域,包括血红蛋白病、肿瘤学、再生医学、心血管疾病、自身免疫疾病和罕见病。2018年,CRISPR Therapeutics将首个CRISPR/Cas9基因编辑疗法推进临床,用于研究镰状细胞病和输血依赖性β地中海贫血的治疗。自2023年底起,CASGEVY®(exagamglogene autotemcel [exa-cel])在多个国家获批,用于治疗符合条件的镰状细胞病或输血依赖性β地中海贫血患者。诺贝尔奖得主的CRISPR技术彻底改变了生物医学研究,代表了一种经过临床验证的强大方法,具有潜力创造一类全新的、可能具有变革性的药物。为了加速和扩大其努力,CRISPR Therapeutics与Vertex Pharmaceuticals等领先公司建立了战略合作伙伴关系。CRISPR Therapeutics AG总部位于瑞士楚格,其全资子公司CRISPR Therapeutics, Inc.位于美国,研发业务则设在美国马萨诸塞州波士顿和加利福尼亚州旧金山。欲了解更多信息,请访问www.crisprtx.com。引用文献:1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380, 2095-1128.+86 21 61207756 info@siriusrna.com媒体联系人:+86 21 61207756 info@siriusrna.com
siRNA引进/卖出基因疗法临床1期临床申请
2025-05-20
·药智网
520这天作为网络情人节,深受情侣们喜爱,如今也成为药企官宣合作的热门日子。今日,一则重磅消息席卷了整个医药圈:辉瑞以高达60.5亿美元的巨额资金引进三生制药的PD-1/VEGF双特异性抗体SSGJ-707,标志着中国创新药出海征程中迎来了又一座意义非凡的新里程碑——成为国内迄今为止首付款金额最高的License deal项目。然而,在这股关注的浪潮之下,今日还有一项创新药出海的合作同样不容忽视,那就是靖因药业与CRISPR Therapeutics的重磅合作,双方将携手推进siRNA创新疗法 的开发,而此次合作的金额也高达8.95亿美元(约合人民币65亿元)。65亿元合作5月20日,靖因药业宣布与CRISPR Therapeutics达成战略合作伙伴关系,聚焦于共同推进siRNA疗法的开发与商业化进程,其背后有着深刻的背景与缘由。图片来源:靖因药业官微靖因药业在siRNA领域深耕已久,拥有自主知识产权的小核酸药物研发核心技术平台,涵盖了靶向递送系统、化学修饰技术以及双链siRNA设计等多个关键环节。以化学修饰技术为例,它能够显著延长siRNA的稳定性和半衰期,从而确保药物在体内的长效作用,这无疑是其在竞争中脱颖而出的重要“武器”。而CRISPR Therapeutics 作为基因治疗领域的佼佼者,具备深厚的研发实力和丰富的技术经验。双方的强强联合,旨在将CRISPR Therapeutics的研发能力与靖因药业的siRNA技术平台有机融合,为血栓及血栓栓塞性疾病患者带来新的希望。根据双方签署的合作协议,合作的核心项目是下一代长效Factor XI(FXI)靶向小干扰RNA(siRNA)疗法SRSD107 ,该药物主要用于治疗血栓及血栓栓塞性疾病。这一药物的研发源于FXI靶点在病理性血栓形成中的关键作用,同时它对正常止血功能的影响相对较小。临床前试验的数据表现令人振奋,单次皮下注射SRSD107后,可使外周血FXI浓度近乎降低100%,并且这一效果能够持续长达半年,同时未观察到明显的出血风险。这使得SRSD107有望成为潜在的同类首创(First-in-Class)和同类最佳(Best-in-Class)新一代更安全的抗凝药物。在合作的商业条款方面,靖因药业将获得CRISPR Therapeutics支付的9500万美元现金及现金等价物作为首付款,并且有资格获得超过8亿美元的预付款和里程碑付款。双方将按照50-50的成本和利润分摊机制共同开发SRSD107。此外,在市场分工上,CRISPR将负责SRSD107在美国的商业化,而靖因药业则将目光投向大中华区市场,致力于该药物在这一地区的推广与销售。此次合作对于靖因药业而言,无疑是一次重大发展机遇。一方面,丰厚的资金支持为其siRNA疗法的研发提供了坚实的物质保障,使其能够更加心无旁骛地投入到创新药物的开发之中;另一方面,与CRISPR Therapeutics这样国际知名的研发机构合作,将有助于提升靖因药业在siRNA疗法领域的研发实力和市场竞争力,进一步丰富和完善其产品管线,为公司的长远发展注入强大动力。对于CRISPR Therapeutics来说,通过与靖因药业的携手合作,能够有效拓展其在心血管疾病领域的产品管线布局。借助靖因药业在siRNA领域的先进技术,CRISPR Therapeutics可以进一步丰富自身的治疗技术平台,从而提升其在医药市场的综合竞争力。值得一提的是,这也是siRNA疗法领域的一个标志性事件,充分展现了行业对于siRNA技术在心血管疾病治疗领域巨大潜力的高度认可。全新路径从心血管疾病到癌症患者,血栓栓塞的阴影无处不在,血栓栓塞性疾病,正悄然成为全球健康的隐匿性“杀手”。数据显示,2023年全球抗凝血剂市场规模达178亿美元,整个抗血栓药物市场更为庞大,2024年营收524亿美元,预计2032年将跃升至964.2亿美元,2025至2032年间年复合增长率高达7.92%。图片来源:https://www.databridgemarketresearch.com/reports/global-antithrombotic-drugs-market然而,传统抗凝治疗的局限性逐渐暴露。抗凝过度引发的出血风险,宛如达摩克利斯之剑,悬于医生与患者心头。在追求疗效与安全的平衡中,siRNA疗法犹如一道曙光,穿透迷雾,为血栓治疗开辟了全新路径。siRNA疗法的出现,是精准医学的完美诠释。它精准锁定凝血因子XI(FXI),在阻断病理性血栓形成的“源头”时,巧妙避开对正常止血功能的干扰。与传统凝血因子Xa抑制剂相比,这种精准打击的优势尤为明显,如同在错综复杂的凝血网络中,精准剪断一根关键线索,而不触碰其他重要环节。其长效性更是一大亮点。临床前及临床试验数据令人惊叹,单次皮下注射SRSD107,就能让外周血FXI浓度近乎归零,且这一效果能维持半年之久。对于患者而言,这不仅减少了频繁给药的痛苦与不便,更提高了治疗的依从性,让规律治疗不再是难事。安全性层面,SRSD107也交出了一份亮眼答卷。在前期试验中,它展现出良好的耐受性,未增加出血风险。siRNA疗法的适应症潜力,更是令人瞩目。从房颤患者的心血管风险,到静脉血栓栓塞症患者的复发隐患;从肿瘤患者的血栓并发症,到血液透析患者的凝血难题;从骨科手术患者围手术期的出血风险,到终末期肾病患者的凝血异常,siRNA疗法都展现出广泛的应用前景,有望成为多种血栓相关疾病患者的福音。创新核酸疗法新星何为靖因药业达成本次合作?这与其深耕创新核酸疗法有关。靖因药业于2021年成立,致力于利用创新核酸疗法来改善人类健康。作为一个新兴的生物技术公司,其在心血管代谢疾病领域展现出巨大的潜力。靖因药业部分在研管线图片来源:药智数据早期,靖因药业将目光锁定于心血管疾病治疗领域,SRSD107注射液应运而生。作为一款凝血因子XI(FXI)靶向小干扰RNA(siRNA)疗法,SRSD107于2023年11月在澳洲提交了首次人体试验(FIH)的申请,仅隔数月,2024年3月便获得了中国CDE的临床试验批准。同年7月,SRSD107在新西兰率先完成Ⅰ期临床试验全部受试者入组给药,再次验证了其在不同种族人群中的安全性和耐受性。2025年3月,靖因药业更是将目光投向欧洲市场,向欧洲药品管理局提交了II期临床试验申请。数据显示,单次皮下注射SRSD107后,外周血FXI浓度近乎被完全敲低,且这一效果能持续长达半年,同时未观察到出血等不良反应。这一突破性的研究成果不仅证明了SRSD107强大的抗凝效果,更凸显了其在安全性方面的显著优势。在SRSD107的研发稳步推进之际,靖因药业并未止步,开启了SRSD101注射液的研发之路,一款用于治疗血脂异常的siRNA药物。2024年,SRSD101完成了在新西兰的Ⅰ期临床试验。SRSD101通过精准靶向PCSK9基因,有效降低LDL-C水平,有望为ASCVD患者提供更优质、更高效的降脂治疗方案。这一创新疗法的研发,不仅丰富了靖因药业的产品矩阵,也进一步巩固了其在心血管疾病治疗领域的领先地位。2025年4月,靖因药业在新药研发领域再次取得突破性进展SRSD216注射液的临床试验申请获美国FDA批准,紧接着在中国的I期临床试验也于4月8日完成首例受试者给药。从SRSD107到SRSD101,再到SRSD216,靖因药业的在研管线涵盖了血栓栓塞性疾病和血脂异常等多个心血管代谢疾病治疗领域,均展现出强大的创新性和临床价值,具有同类首创或同类最佳潜质,有望在未来市场竞争中脱颖而出,重塑心血管疾病治疗格局。#520 #创新药出海 #靖因药业 #siRNA创新疗法 #SRSD107 #SRSD101 #SRSD216参考来源:1.药智数据2.https://mp.weixin.qq.com/s/VYIrcJ_fZKVF1UlzM17GrA3.https://www.gminsights.com/industry-analysis/thrombosis-drugs-market4.https://www.databridgemarketresearch.com/reports/global-antithrombotic-drugs-market5.https://ir.crisprtx.com/news-releases/news-release-details/crispr-therapeutics-and-sirius-therapeutics-announce-multi6.https://www.globenewswire.com/news-release/2025/05/19/3084420/0/en/CRISPR-Therapeutics-and-Sirius-Therapeutics-Announce-Multi-Target-Collaboration-to-Develop-Novel-siRNA-Therapies.html声明:本内容仅用作医药行业信息传播,为作者独立观点,不代表药智网立场。如需转载,请务必注明文章作者和来源。对本文有异议或投诉,请联系maxuelian@yaozh.com。责任编辑 | 史蒂文合作、投稿、转载开白 | 马老师 18323856316(同微信) 阅读原文,是受欢迎的文章哦
siRNA引进/卖出基因疗法临床申请核酸药物
2025-05-20
-Collaboration brings together complementary capabilities to co-develop and co-commercialize SRSD107, a next generation, long-acting Factor XI (FXI)
small interfering RNA (siRNA) for the treatment of thromboembolic disorders-
-SRSD107 demonstrated peak reductions in FXI activity >93% and increases in activated partial thromboplastin time (aPTT) >2x with maintained efficacy up to 6 months post-dosing in a Phase 1 clinical trial-
-Under the agreement, CRISPR Therapeutics will make an upfront payment of $25 million in cash and $70 million in equity to Sirius Therapeutics; CRISPR Therapeutics also has rights to exclusively license up to two additional siRNA programs-
-Expands CRISPR’s therapeutic toolkit to develop a broader range of transformative gene-based medicines in addition to the gene-editing programs in the clinic-
ZUG, Switzerland and BOSTON, MA, USA and SAN DIEGO, CA, USA and SHANGHAI, China I May 19, 2025 I
CRISPR Therapeutics (NASDAQ: CRSP), a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases, and Sirius Therapeutics, a clinical stage biotech company developing innovative small interfering RNA (siRNA) therapies for global markets, today announced a strategic partnership to develop and commercialize siRNA therapies.
“We are excited to partner with Sirius, and broaden our cardiovascular medicine portfolio, on the heels of promising top-line data that we recently shared for CTX310, which targets ANGPTL3,” said Samarth Kulkarni, Ph.D., Chairman and Chief Executive Officer of CRISPR Therapeutics. “Coagulation Factor XI represents an innovative and highly compelling target for treating thrombotic diseases that affect millions worldwide. SRSD107, which targets Factor XI, has the potential to be a best-in-class therapy, offering infrequent dosing and improved patient outcomes. Sirius’ siRNA platform complements our existing capabilities and expands our therapeutic toolkit, enabling us to develop a broader range of transformative gene-based medicines.”
“We are pleased to collaborate with CRISPR Therapeutics, a recognized leader in the development of gene-based medicines,” said Qunsheng Ji, MD, Ph.D. Chief Executive Officer of Sirius Therapeutics. “Thrombotic diseases represent a significant unmet need, and our promising Phase 1 data highlights the potential of SRSD107 as a best-in-class Factor XI-targeted therapy. Sirius is committed to addressing the needs of these patients, as we work with CRISPR Therapeutics to advance novel siRNA therapies globally.”
“There is a large population of patients who are at risk for potentially life-threatening thromboembolic events due to underlying co-morbid diseases such as malignancy, cardiovascular disease, and hyper-coagulability. A significant percentage of these patients are inadequately treated due to concerns for bleeding risk, or challenges with compliance,” said Christian T. Ruff, M.D., M.P.H., senior investigator of TIMI Group, director General Cardiology, Brigham and Women’s Hospital, and associate professor, Harvard Medical School. “SRSD107 offers the potential for a therapy with lower bleeding risk, infrequent dosing for better compliance, without concerns for renal clearance or drug interactions, and reversibility to further mitigate bleeding risks that could be differentiated from currently available therapies and other Factor XI modalities.”
SRSD107 is a next generation, long-acting siRNA designed to selectively inhibit Factor XI (FXI), a key driver of pathological thrombosis with minimal impact on normal hemostasis. By targeting FXI, SRSD107 aims to reduce thrombotic events while minimizing the risk of bleeding – representing a differentiated approach compared to Factor Xa inhibitors. In addition, SRSD107 may offer the potential for reversibility not observed with other anti-Factor XI modalities. The addressable population includes patients with atrial fibrillation, venous thromboembolism (VTE), cancer-associated thrombosis, chronic Coronary Artery Disease (CAD), chronic Peripheral Vascular Disease (PVD), end-stage renal disease requiring hemodialysis, and patients undergoing major orthopedic surgery, where bleeding risk limits existing therapies.
The clinical program for SRSD107 includes two promising Phase 1 clinical trials, where single doses of SRSD107 were found to be safe and well tolerated. In addition, SRSD107 demonstrated robust pharmacodynamic effects, including reductions of over 93% in FXI levels and FXI activity (FXIa), along with more than a twofold increase in activated partial thromboplastin time (aPTT) relative to baseline. These effects were sustained, with responses maintained for up to 6 months post-dosing. SRSD107 has the potential to be a best-in-class FXI inhibitor, showing deep reductions in FXI via semi-annual subcutaneous injection. Results from the Phase 1 trials were presented at both the 2025 Annual Scientific Sessions of the American College of Cardiology and the 2024 Annual Meeting of the American Society of Hematology.
Figure 1. SRSD107 Phase 1 Clinical Results: Sustained, dose-dependent pharmacodynamic response to therapy
A Phase 2 clinical trial of SRSD107 is being initiated to evaluate its safety and efficacy for the prevention of VTE in patients undergoing total knee arthroplasty. The trial aims to confirm the anticoagulant benefits of SRSD107 and to inform dose selection for future pivotal trials.
Collaboration Details
Under the terms of the agreement, CRISPR Therapeutics will make an upfront payment of $25 million in cash and $70 million in equity to Sirius Therapeutics. The companies will jointly develop SRSD107 under a 50-50 cost and profit-sharing structure. CRISPR Therapeutics will lead commercialization in the U.S., while Sirius will be responsible for commercialization in Greater China.
Additionally, CRISPR Therapeutics will have the option to nominate up to two siRNA targets for research and development. For each target, CRISPR Therapeutics will fund research and retain opt-in rights to lead clinical development and commercialization. Sirius will be eligible to receive milestone payments, as well as tiered royalties ranging from high single to low-double digits.
About CRISPR Therapeutics
Since its inception over a decade ago, CRISPR Therapeutics has evolved from a research-stage company advancing gene editing programs into a leader that celebrated the historic approval of the first-ever CRISPR-based therapy. The Company has a diverse portfolio of product candidates across a broad range of disease areas including hemoglobinopathies, oncology, regenerative medicine, cardiovascular, autoimmune, and rare diseases. In 2018, CRISPR Therapeutics advanced the first-ever CRISPR/Cas9 gene-edited therapy into the clinic to investigate the treatment of sickle cell disease and transfusion-dependent beta thalassemia. Beginning in late 2023, CASGEVY
®
(exagamglogene autotemcel [exa-cel]) was approved in several countries to treat eligible patients with either of these conditions. The Nobel Prize-winning CRISPR technology has revolutionized biomedical research and represents a powerful, clinically validated approach with the potential to create a new class of potentially transformative medicines. To accelerate and expand its efforts, CRISPR Therapeutics has formed strategic partnerships with leading companies including Vertex Pharmaceuticals. CRISPR Therapeutics AG is headquartered in Zug, Switzerland, with its wholly-owned U.S. subsidiary, CRISPR Therapeutics, Inc., and R&D operations based in Boston, Massachusetts and San Francisco, California. To learn more, visit
www.crisprtx.com
.
CRISPR THERAPEUTICS
®
standard character mark and design logo, CTX310™ and CTX320™ are trademarks and registered trademarks of CRISPR Therapeutics AG. CASGEVY
®
and the CASGEVY logo are registered trademarks of Vertex Pharmaceuticals Incorporated. All other trademarks and registered trademarks are the property of their respective owners.
About Thromboembolic Disorders
Thrombosis, or blood clot formation, is the common underlying mechanism of most cases of myocardial infarction, ischemic stroke, and venous thromboembolism. According to a trial in The Lancet
1
of regional and global mortality rates, thromboembolic disorders are estimated to cause as many as 1 in 4 deaths worldwide.
About SRSD107
SRSD107 is a novel double-stranded small interfering ribonucleic acid (siRNA). SRSD107 specifically targets the human coagulation factor XI (FXI) mRNA and inhibits FXI protein expression, thereby blocking the intrinsic coagulation pathway and promoting anticoagulant/anti-thrombotic effects. SRSD107 has been engineered for the potential to enable twice-a-year dosing.
About Sirius Therapeutics
Sirius is a clinical stage biotech company developing innovative siRNA therapies for global markets. We are dedicated to discovering and developing new treatment options for cardiovascular and cerebrovascular disease and translating siRNA technology into transformative medicine for chronic disease patients. Sirius’s most advanced products are SRSD107 for the treatment of thromboembolic disorders, SRSD216 for the treatment of hyperlipoproteinemia, and SRSD101 for the treatment of dyslipidemia.
Founded in 2021 by a world-class leadership team and investors, Sirius has established an innovation center in the United States and translational medicine center in China. Sirius has raised nearly US$150 million funding to date from OrbiMed, Creacion Ventures, Hankang Capital, Delos Capital, and BioTrack Capital. Learn more at
www.siriusrna.com
References:
1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380, 2095-1128.
SOURCE:
CRISPR Therapeutics
临床结果临床1期引进/卖出上市批准临床2期
100 项与 SRSD-101 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 原发性高胆固醇血症 | 临床申请批准 | 中国 | 2023-11-15 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用