预约演示
更新于:2025-01-11
Trimethoprim Hydrochloride
盐酸甲氧苄啶
更新于:2025-01-11
概要
基本信息
结构/序列
分子式C14H19ClN4O3 |
InChIKeyYLCCEQZHUHUYPA-UHFFFAOYSA-N |
CAS号60834-30-2 |
关联
1
项与 盐酸甲氧苄啶 相关的临床试验NCT03424525
11C-Trimethoprim PET/CT Imaging to Evaluate Biodistribution and Kinetics in Human
Patients with suspected bacterial infection at the time screening are eligible for this study. Patients may participate in this study if they are at least 18 years of age, and most participants will be receiving care at the clinical practices of the University of Pennsylvania. Up to 30 subjects will participate in two different imaging cohorts.
The Biodistribution cohort will include up to 5 patients referred from orthopedics who will undergo a series of vertex to mid-thigh (or feet if indicated) biodistribution [11C]trimethoprim PET/CT scans over a period of approximately 2 ½ hours.
The Dynamic cohort will include up to 25 patients who will undergo approximately 60 minutes of dynamic scanning followed by up to 2 static skull base to mid-thigh (or feet if indicated) scans imaging post injection of [11C]trimethoprim. Some subjects who may be selected clinically to undergo surgical or antibiotic treatment may undergo a second therapy may also undergo an optional second [11C]trimethoprim PET/CT after the initiation of therapy to collect pilot data on the changes in [11C]trimethoprim biodistribution and uptake with therapy, the timing of this scan may vary depending on the type of treatment the patient is receiving.
Patients will also undergo baseline lab tests complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and blood cultures. If these tests are done as part of clinical standard of care they will not need to be repeated for this study.
The Biodistribution cohort will include up to 5 patients referred from orthopedics who will undergo a series of vertex to mid-thigh (or feet if indicated) biodistribution [11C]trimethoprim PET/CT scans over a period of approximately 2 ½ hours.
The Dynamic cohort will include up to 25 patients who will undergo approximately 60 minutes of dynamic scanning followed by up to 2 static skull base to mid-thigh (or feet if indicated) scans imaging post injection of [11C]trimethoprim. Some subjects who may be selected clinically to undergo surgical or antibiotic treatment may undergo a second therapy may also undergo an optional second [11C]trimethoprim PET/CT after the initiation of therapy to collect pilot data on the changes in [11C]trimethoprim biodistribution and uptake with therapy, the timing of this scan may vary depending on the type of treatment the patient is receiving.
Patients will also undergo baseline lab tests complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) and blood cultures. If these tests are done as part of clinical standard of care they will not need to be repeated for this study.
开始日期2018-02-01 |
申办/合作机构 |
100 项与 盐酸甲氧苄啶 相关的临床结果
登录后查看更多信息
100 项与 盐酸甲氧苄啶 相关的转化医学
登录后查看更多信息
100 项与 盐酸甲氧苄啶 相关的专利(医药)
登录后查看更多信息
18
项与 盐酸甲氧苄啶 相关的文献(医药)2025-03-01·TALANTA
Voltammetric methodology for the quality control and monitoring of sulfamethoxazole removal from water
Article
作者: Sanromán, M Ángeles ; González-Romero, Elisa ; Pazos, Marta ; Bernárdez, Nuria ; Caruncho-Pérez, Sara
Sulfamethoxazole is an antibiotic that is among the drugs most frequently found in waters around the world because of its habitual consumption and its high chemical stability that prevents it from being eliminated from the environment. In this study, an electroanalytical methodology based on differential pulse voltammetry is developed for the analysis of sulfamethoxazole at trace levels in water. After the optimization of the instrumental parameters a linear range from 6.59 to 96.27 μM was found with limits of detection and quantification of 1.98 and 6.59 μM, respectively, with an RSD below 6 %. Moreover, several validation studies involving different pH values, water samples and instrumentation-techniques were performed in order to ensure the robustness of the method. For this purpose, the peak area was used as quantitative variable since it is not affected by the pH of the medium even if there is any modification of this parameter during the experiments. Furthermore, the effect of other drug such as trimethoprim on the analytical signal of sulfamethoxazole was also evaluated. Once the method was developed it was tested on the quality control of Soltrim®, obtaining recoveries between 98 and 102 %. Lastly, the voltammetric method was applied for the in situ monitoring of sulfamethoxazole's removal from water samples, specifically by anodic oxidation and electro-Fenton treatments. While the former was coupled to an adsorption process, the latter was carried out with different iron sources including commercial medicines that can be found in wastewater. The problem of significant variation in pH during the treatment was solved by working with the peak area, and so obtaining valid and reliable kinetic data. Although anodic oxidation proved to be faster considering the calculated kobs, electro-Fenton turned out to be more efficient in eliminating the drug, achieving the disappearance of its analytical signal in only 30 min of treatment.
2025-02-01·JOURNAL OF FISH DISEASES
Antimicrobial Susceptibility and Local Epidemiological Cut‐Off Values of Vibrio anguillarum Isolated From Fish Farms in Turkey
Article
作者: Concha, Christopher ; Satıcıoğlu, Izzet Burçin ; Altun, Soner ; Balcı, Kübra ; Duman, Muhammed ; Miranda, Claudio D. ; Bayrak, Nisa ; Avendaño‐Herrera, Ruben ; Taşgın, Merve
ABSTRACT:
Studies on preventing antimicrobial resistance in aquaculture emphasise the need to responsibly and prudently use antimicrobials, selecting those most effective in controlling and/or reducing mortalities caused by vibriosis. In this study, the distribution of the antimicrobial susceptibility of 28 Vibrio anguillarum isolates from Turkish fish farms was determined using the broth microdilution minimum inhibitory concentration (MIC) test according to the Clinical and Laboratory Standards Institute (CLSI) guideline. The epidemiological cut‐off (COWT) values of the V. anguillarum isolates of florfenicol (FLO), tetracycline (TET), doxycycline (DOX), oxolinic acid (OXO), enrofloxacin (ENR) and trimethoprim/sulfamethoxazole (SXT) were calculated using the normalised resistance interpretation (NRI) and ECOFFinder methods. Isolates were categorised as belonging to the fully susceptible wild‐type (WT) or non‐wild‐type (NWT) populations. Calculated COWT values (in μg mL−1) were ≤ 1.0 for FLO and DOX, ≤ 0.5 for TET, ≤ 0.016 for ENR, ≤ 0.032 for OXO, and ≤ 4.0 for SXT using the NRI analysis. Percentages of V. anguillarum isolates categorised as belonging to the NWT population were small for the antimicrobials FLO (10.7%) and SXT (0%), whereas they were higher for the antimicrobials OXO (39.3%) and ENR (39.3%). This is the first study to estimate the local COWT values for antibiotics used in the control of V. anguillarum isolates recovered from farmed fish in Turkey.
2024-12-01·EUROPEAN JOURNAL OF CLINICAL MICROBIOLOGY & INFECTIOUS DISEASES
Chromobacterium violaceum infections in children: two case reports and literature review
Review
作者: Ye, Sheng ; Ren, YiFan ; Jiang, ZhiHong
PURPOSE:
Chromobacterium violaceum(C. violaceum) is a gram-negative bacterium that rarely infects humans, especially children. However, the mortality rate is high and there are no clear guidelines for treatment. The aim of this paper is to increase clinicians' awareness of diseases caused by C. violaceum infections in children, to diagnose and treat them in a timely manner, to improve patient survival and to reduce mortality.
RESULTS:
We analysed the latest paediatric-related English language literature over the last 10 years and summarised the latest mechanisms of injury, susceptibility factors, adverse prognostic and mortality predictors, mortality rates, methods to reduce mortality, clinical manifestations, new diagnostic methods, therapeutic agents and directions for future drug development for C. violaceum.
CONCLUSIONS:
Based on the available data, we conclude that the possibility of C. violaceum infection should be considered and diagnosed when cellulitis, septicaemia and visceral abscesses develop in children with a history of skin injury and exposure to stagnant water or soil. When clinicians strongly suspect that a child is infected with this bacterium, the recommended medication is ciprofloxacin if the child presents with severe illness. If the child has a non-severe condition, medications with relatively fewer side effects for children can be chosen, such as gentamicin, trimethoprim/ sulfamethoxazole, imipenem, and other drugs. The physician can then adjust the antimicrobial regimen based on the antimicrobial spectrum after obtaining the drug sensitivity results.
100 项与 盐酸甲氧苄啶 相关的药物交易
登录后查看更多信息
研发状态
10 条最早获批的记录, 后查看更多信息
登录
| 适应症 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|
| 中耳炎 | 美国 | 1995-06-23 | |
| 泌尿道感染 | 美国 | 1995-06-23 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
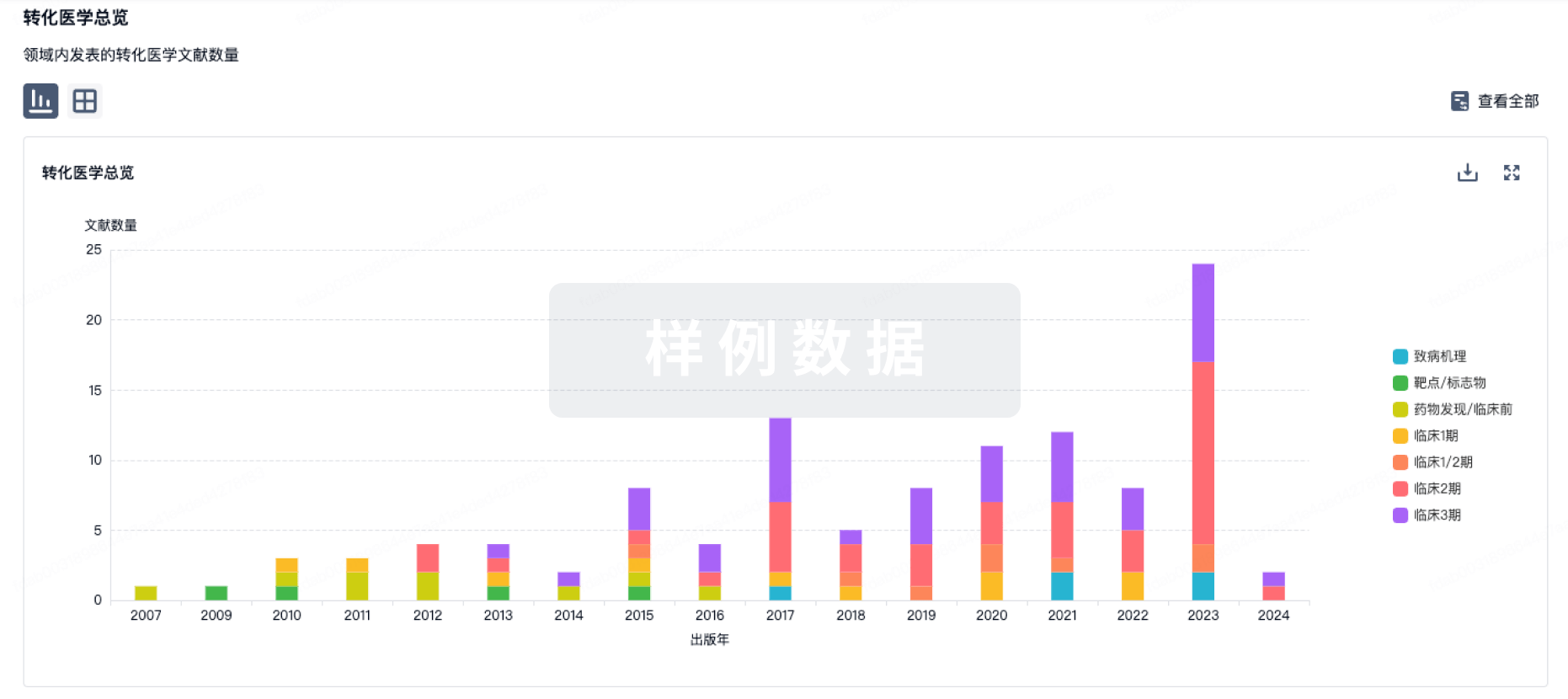
转化医学
使用我们的转化医学数据加速您的研究。
登录
或
药物交易
使用我们的药物交易数据加速您的研究。
登录
或
核心专利
使用我们的核心专利数据促进您的研究。
登录
或
临床分析
紧跟全球注册中心的最新临床试验。
登录
或
批准
利用最新的监管批准信息加速您的研究。
登录
或
特殊审评
只需点击几下即可了解关键药物信息。
登录
或
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

