3
项与 Regulatory T-cells(Dana-Farber Cancer Institute) 相关的临床试验A Pilot/Phase 1 Study of Immunosuppression-free Regulatory T-cell Graft-engineered Haploidentical Hematopoietic Cell Transplantation in Relapsed/Refractory and Ultra-High-risk AML/MDS
This research study is evaluating the safety and efficacy of the IS-free Treg-cell graft-engineered haplo transplant method in people with relapsed/refractory and Ultra-high risk acute myeloid leukemia (AML) and/or myelodysplastic syndromes (MDS) receiving a haploidentical donor allogeneic hematopoietic stem cell transplant (HSCT).
The names of the study interventions involved in this study are:
* Radiation-Total Myeloid and Lymphoid Irradiation (TMLI
* Chemotherapy (Fludarabine, Thiotepa, Cyclophosphamide plus Mesna)
* Infusion of haplo Treg-enriched donor cells (experimental therapy)
* Infusion of unmodified haplo donor T cells (includes cancer-fighting T effector cells)
* Infusion of haplo donor CD34+ Peripheral Blood Stem Cells
Renal Transplantation Followed By Infusion of T-Regulatory Cells Made With Belatacept Ex-Vivo
This research study is for patients who are going to receive a kidney transplant from a living donor. After kidney transplantation, it is necessary for transplant recipients to take "immunosuppressive drugs". These drugs work by preventing the body's immune cells from attacking and "rejecting" the new kidney. Taking these drugs long-term may also cause harm to the transplanted kidney. Therefore, the transplant community is very interested in finding ways to decrease immunosuppressive drug treatment and further reduce the risk of kidney rejection. One method to do so is known as "induction of tolerance", which is when the person who receives a transplant has treatment to make their immune cells tolerant to the donor cells.
In this study, we will try to induce tolerance by mixing recipient cells and their donor's cells together with belatacept, an immunosuppressive drug. Belatacept is a protein that attaches to immune system cells, interferes with the immune response and results in tolerance induction.
After we mix the recipient cells with the donor's cells, we will sort out one particular kind of immune cell, called a regulatory T cell, and inject them back into the recipient. Regulatory T cells are the cells that are affected by induction to reduce rejection of donated organs. This method for inducing tolerance has been used in bone marrow transplantation, but this is the first time it is being done in kidney transplantation.
This study is being conducted as part of a unique collaboration of US and EU centers called The ONE Study. The ONE Study centers have agreed to work together using common protocols and procedures but with each testing their own regulatory population for safety and the ability to promote kidney survival. Sharing data among the participating sites will permit a deeper understanding of how and why some treatments might succeed while others work less well.
/ Active, not recruiting临床1期IIT A Phase I Trial of Regulatory T-cells Plus Low-Dose Interleukin-2 for Steroid-Refractory Chronic Graft-versus-Host-Disease
This research study is a Phase I clinical trial, which tests the safety of an investigational combination of IL-2 plus donor anti-inflammatory Treg cells and also tries to define the appropriate dose of the investigational combination of IL-2 plus donor anti-inflammatory Treg cells to use for further studies. IL-2 is involved with cell signaling and regulation of white blood cells (WBCs). WBCs are part of the immune system. Treg cells are also part of the immune system; they are involved with anti-inflammatory responses. "Investigational" means that the combination of IL-2 and anti-inflammatory Treg cell infusion is being studied. It also means that the FDA (U.S. Food and Drug Administration) has not approved the combination of IL-2 and anti-inflammatory Treg cell infusion for use in people with cGVHD.
Chronic GVHD is a medical condition that may occur after you have received your bone marrow, stem cell or cord blood transplant from a donor. The donor's immune system may recognize your body (the host) as foreign and attempt to 'reject' it. This process is known as graft-versus-host disease.
Traditional standard therapy to treat cGVHD is prednisone (steroids). Participants on this trial have not responded to steroid therapy. The investigators are looking to assess the safety and optimal dose for the combination of IL-2 plus donor anti-inflammatory Treg cells, that may help control cGVHD by stopping the donor's immune system from 'rejecting' your body.
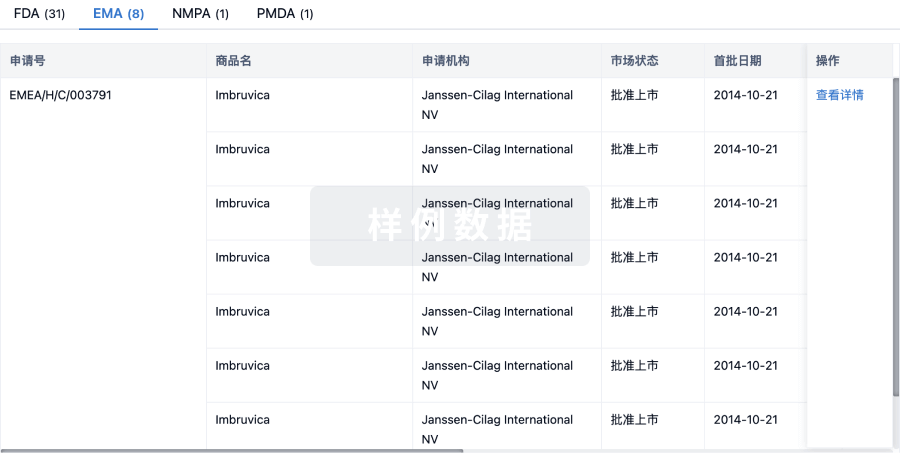
100 项与 Regulatory T-cells(Dana-Farber Cancer Institute) 相关的临床结果
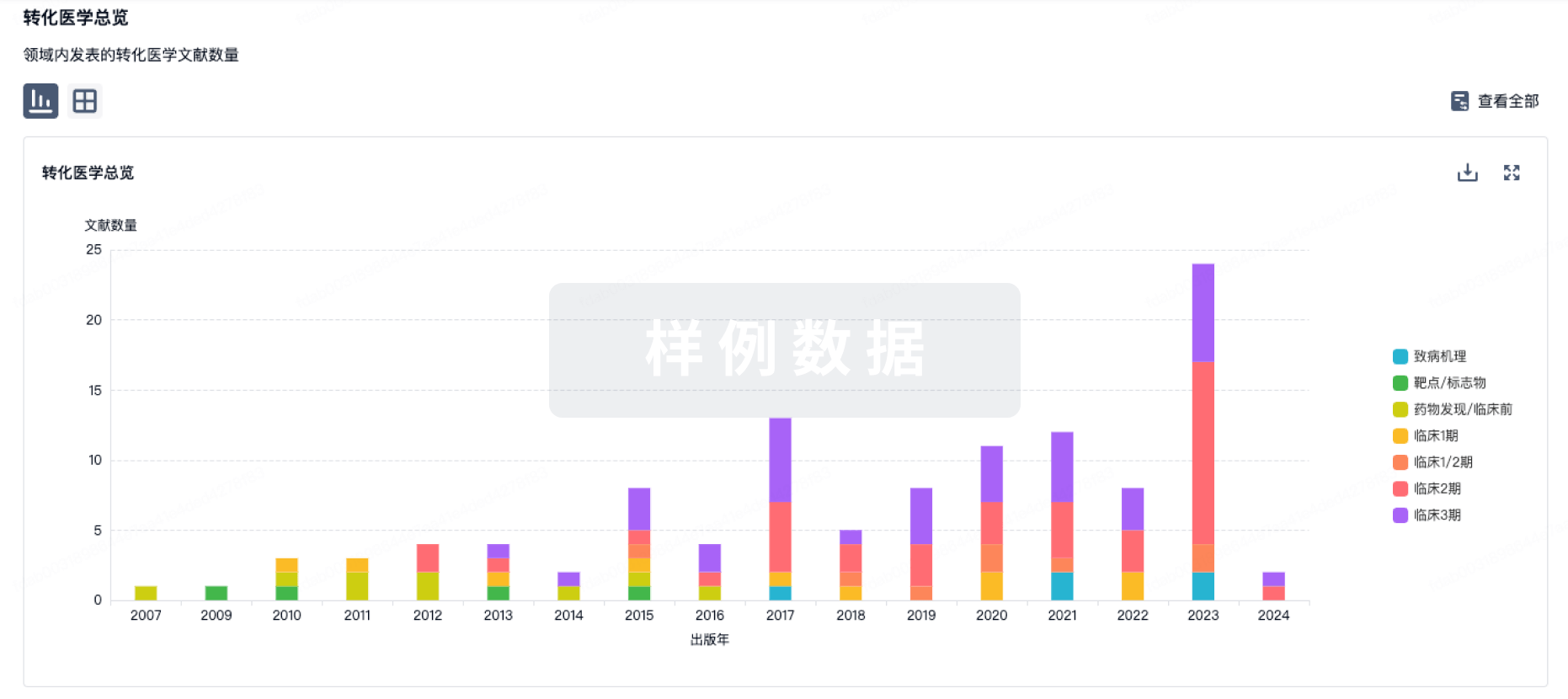
100 项与 Regulatory T-cells(Dana-Farber Cancer Institute) 相关的转化医学
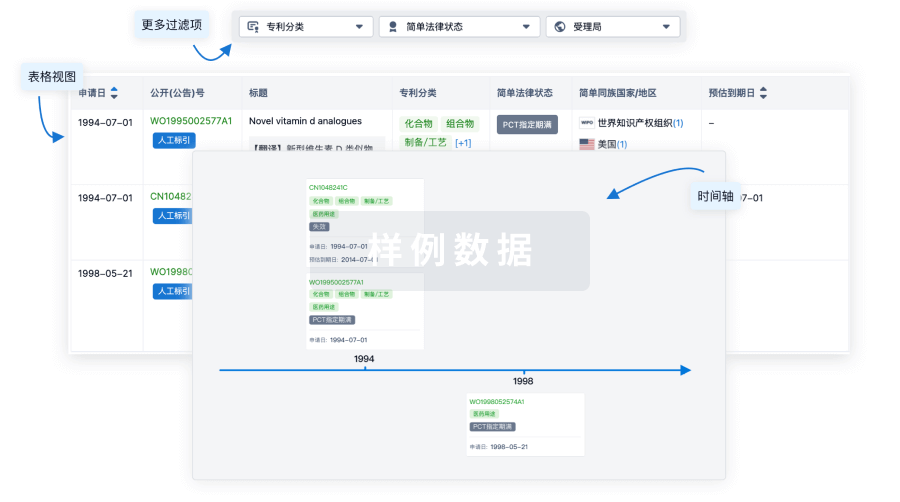
100 项与 Regulatory T-cells(Dana-Farber Cancer Institute) 相关的专利(医药)
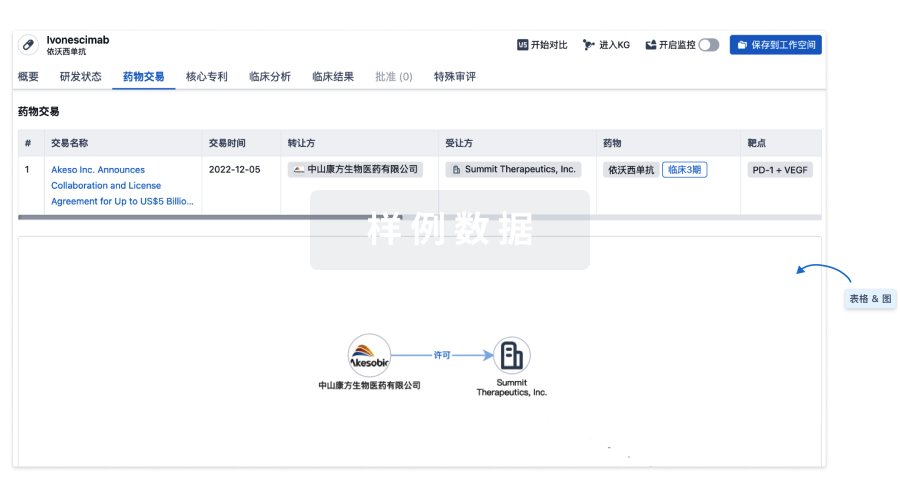
100 项与 Regulatory T-cells(Dana-Farber Cancer Institute) 相关的药物交易