预约演示
更新于:2025-08-30
Napa9-SPG
更新于:2025-08-30
概要
基本信息
非在研机构- |
权益机构- |
最高研发阶段临床前 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评- |
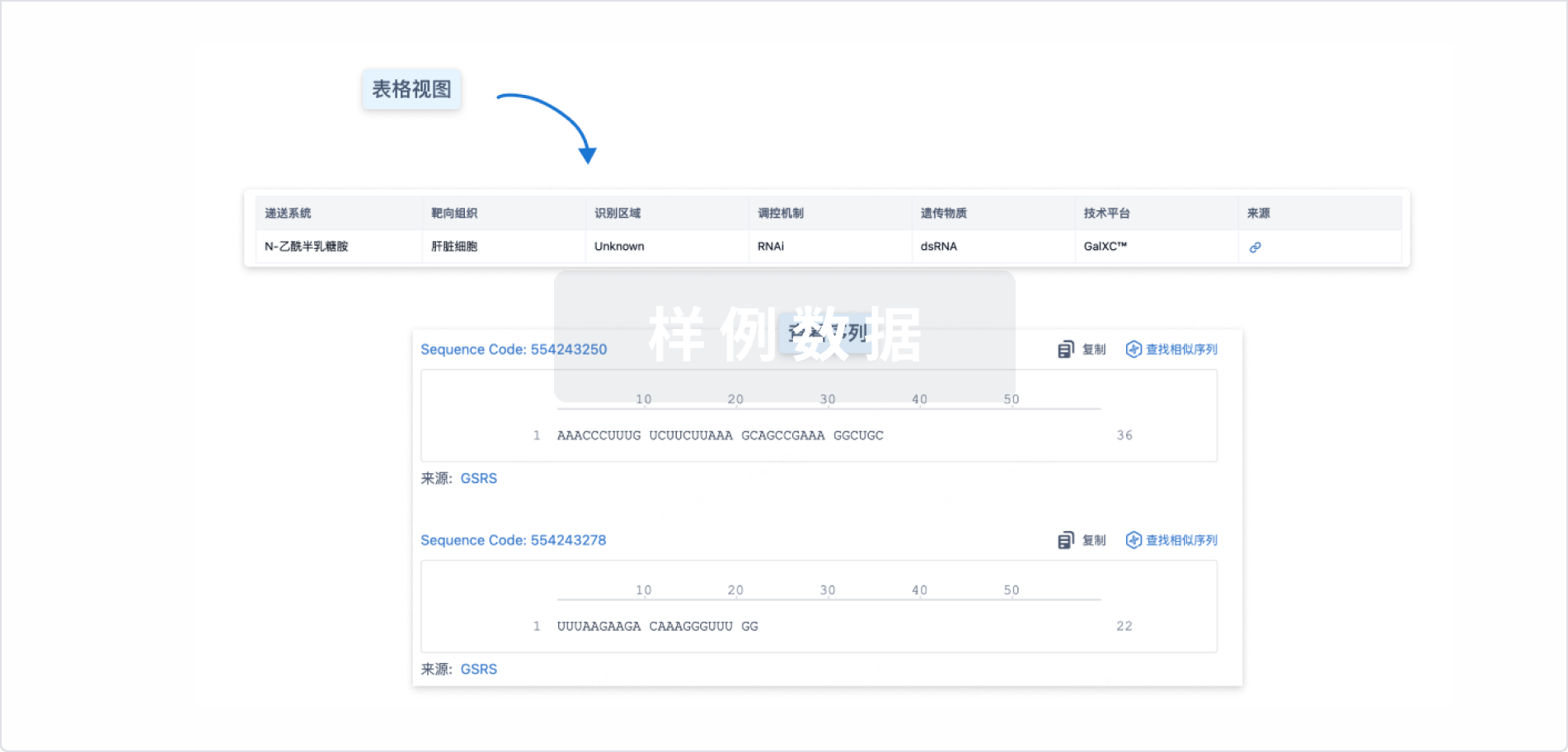
结构/序列
使用我们的RNA技术数据为新药研发加速。
登录
或
关联
100 项与 Napa9-SPG 相关的临床结果
登录后查看更多信息
100 项与 Napa9-SPG 相关的转化医学
登录后查看更多信息
100 项与 Napa9-SPG 相关的专利(医药)
登录后查看更多信息
38
项与 Napa9-SPG 相关的文献(医药)2025-12-31·Human Vaccines & Immunotherapeutics
PfCSP-ferritin nanoparticle malaria vaccine antigen formulated with aluminum-salt and CpG 1018® adjuvants: Preformulation characterization, antigen-adjuvant interactions, and mouse immunogenicity studies
Article
作者: Julien, Jean-Philippe ; Hickey, John M. ; Doering, Jennifer ; Sharma, Nitya ; Costa, Giulia ; Volkin, David B. ; Wizel, Benjamin ; Joshi, Sangeeta B. ; Prieto, Katherine ; Fairlamb, Max ; Levashina, Elena A. ; Mantis, Nicholas J. ; Adewunmi, Yetunde
Circumsporozite protein (CSP), the most abundant surface protein in parasitic Plasmodium falciparum (Pf) sporozoite and an attractive target for malaria vaccine design, has been shown to induce protective humoral response in humans. In this work, we characterized and formulated a promising recombinant PfCSP immunogen (155) candidate consisting of two PfCSP epitopes (i.e. junction, NANP repeat) fused to H. pylori apoferritin forming a 24-mer nanoparticle. In addition, two N-linked glycans were engineered to mitigate possible anti-apoferritin immune responses, and a universal T-cell epitope was included to further enhance immunogenicity. Physicochemical characterization of the 155 antigen was performed including primary structure, post-translational modifications, conformational stability, and particle size. A competitive ELISA was developed to assess antigen binding to a PfCSP-specific mAb. The in vitro antigenicity of the 155 antigen was measured upon formulation with adjuvants, including aluminum-salts (i.e. AlhydrogelTM, Adju-PhosTM) and the TLR-9 agonist CpG 1018®, when freshly combined and after storage at different temperatures over 3 months. The in vivo immunological impact of various adjuvanted formulations of the 155 antigen was investigated in mice. The results support the formulation of 155 with AlhydrogelTM + CpG 1018® adjuvants as a promising recombinant malaria vaccine candidate from both a pharmaceutical and immunological perspective.
2025-08-01·JOURNAL OF CONTROLLED RELEASE
Broadly active intranasal influenza vaccine with a nanocomplex particulate adjuvant targeting mast cells and toll-like receptor 9
Article
作者: Williamson, Grace L ; Staats, Herman F ; Lukesh, Nicole R ; Petrovsky, Nikolai ; Hendy, Dylan A ; Ashcraft, Kathleen A ; Abraham, Mathew A ; Ross, Ted M ; Bachelder, Eric M ; Heise, Mark T ; Landon, Chelsea D ; Murphy, Connor T ; Pena, Erik S ; Carlock, Michael ; Abraham, Soman N ; Ontiveros-Padilla, Luis ; Ainslie, Kristy M
Flumist is the only FDA-approved intranasal influenza vaccine. Although it has recently been approved for at-home use, it has significant limitations. These include reduced effectiveness in generating a protective immune response in patients with extensive influenza exposure, safety concerns due to its live attenuated virus formulation, and reduced efficacy due to viral drift/shift. To address this limitation, we have developed a nanocomplex comprised of a mast cell (MC) agonist and toll-like receptor 9 (TLR9) ligand to adjuvant a broadly acting influenza antigen. The newly reported MC agonist was identified by screening mastoparan-7 analogs for MC degranulation activity, which led to a more active peptide analog, MP12W. Positively charged MP12W spontaneously forms nanoparticulate complexes (NPs) with CpG 1826 that were then used to intranasally vaccinate mice with a computationally optimized broadly reactive antigen (COBRA) hemagglutinin (HA) protein. The NPs were further optimized by substituting CpG 1826 with CpG 55.2, a TLR-9 agonist identified by machine learning to be more active in humans. MP12W-CpG 1826 NPs showed an increased pro-inflammatory response and decreased cytotoxicity in vitro compared to M7 complexes, translating into a safer profile in a model of increased hypersensitivity, collaborative cross mice 027 (CC027). Intranasal vaccination with this complex and broadly reactive HA resulted in higher mucosal antibody concentration and increased cytokine production with antigen recall. These responses were enhanced with MP12W-CpG 55.2 NP vaccination. MP12W-CpG NPs provided similar protection in an influenza challenge model. This study demonstrates the potential of this novel intranasal nanocomplex for vaccination.
2023-01-01·Journal of inflammation research
Electroacupuncture Alleviates Lung Injury in CpG1826-Challenged Mice via Modulating CD39-NLRP3 Pathway.
Article
作者: Hu, Xiumin ; Wu, Jiasi ; Xiong, Xin
Purpose:
Cytokine storm secondary lung injury (CSSLI) is the leading death cause in COVID-19 virus infection, and CD39-dominated purinergic brake drives NLRP3 inflammasome activation and pyroptosis, which plays a crucial role in the pathogenesis of CSSLI. Though electroacupuncture (EA) can alleviate lung injury caused by a variety of inducers, its effect on CSSLI and the underlying mechanism needs further investigation.
Methods:
We established a widely recognized CSSLI mice model with CpG1826 (CpG), a TLR-9 agonist agent. Luminex liquid chip was employed to detect serum levels of 12 cytokines/chemokines to evaluate cytokine storm formation. H+E staining and transmission electron microscope were applied to examine pulmonary pathological injury and alveolar macrophage structure, respectively. IL-1β, IL-18, IL-1α, and HMGB-1 in BAL fluid were determined by ELISA kits. mRNA and protein levels of lung CD39 and NLRP3 were assessed by qRT-PCR and Western blotting. An in vitro model was also established by incubating PMA-differentiated THP-1 cells with serum samples obtained from relevant group of mice.
Results:
Repeated CpG induced CSSLI together with the elevation of 11 cytokines/chemokines including GM-CSF, IL-16, IL-1α, MCP-1, IL-2, IL-10, CCL3, IL-1β, TNF-α, IL-6, and IL-17A, though not IFN-γ, which was reduced by EA pretreatment to a different extent. EA also alleviated lung injury and recovered lung macrophage structure. Moreover, CpG enhanced IL-1β and IL-18 level in BAL fluid, promoted NLRP3, while suppressing CD39 expression in lung, all of which were reversed by EA pretreatment. Of note, EA failed to further decrease BAL fluid IL-1β, IL-18, IL-1α, and HMGB-1 levels when A438079, a selective inhibitor of P2X7, was administered. However, both CD39 and NLRP3 are dispensable for EA decreasing multi-cytokine secretion in serum-incubated and CpG-stimulated THP-1 cells. Taken together, EA alleviated CSSLI in CpG-challenged mice by regulating the CD39-NLRP3 pathway in a P2X7-dependent way.
Conclusion:
EA demonstrated potential to be applied in COVID-19 treatment.
5
项与 Napa9-SPG 相关的新闻(医药)2024-03-07
DENVER--(
BUSINESS WIRE
)--
TriSalus Life Sciences
®
Inc.
(Nasdaq: TLSI), an oncology company integrating its novel delivery technology with immunotherapy to transform treatment for patients with liver and pancreatic tumors, today announced that the International Nonproprietary Names (INN) Expert Committee of the World Health Organization (WHO) and the United States Adopted Names (USAN) Council have approved the use of the nonproprietary name of "
nelitolimod
" for SD-101, a class C TLR-9 agonist. Nelitolimod is the Company’s novel lead drug candidate that is currently being studied in three Phase 1/1b trials for the treatment of uveal melanoma with liver metastases, hepatocellular carcinoma, intrahepatic cholangiocarcinoma, and locally advanced pancreatic ductal adenocarcinoma.
“The WHO INN and USAN approval of nelitolimod is an important milestone in the continued progress we are making with our nelitolimod program,” said Mary Szela, Chief Executive Officer and President of TriSalus. “This accomplishment, along with the recent assignment of a new technology HCPCS Code for our TriNav
®
Infusion System, further positions TriSalus to deliver on our mission to overcome key treatment barriers in liver and pancreatic tumors and make a meaningful difference in the lives of patients suffering from cancer.”
TriSalus’ unique approach leverages its innovative delivery device together with its immunotherapeutic drug to overcome the mechanical and biologic barriers present in the tumor microenvironment. This approach has the potential to enable more durable responses by patients to other immunotherapeutics, thereby facilitating better patient outcomes. Data from TriSalus’ Phase 1/1b trials indicates that the Company’s approach in liver and pancreatic tumors is well tolerated with encouraging efficacy and immune signals, with evidence of nelitolimod being delivered by the TriNav system into difficult to reach tumors, potentially overcoming limitations posed by intravenous or direct needle injection approaches.
Information on nelitolimod will be posted on the USAN website (
www.ama-assn.org/go/usan
) and will be published in the Chemical Abstracts Service and in the U.S. Pharmacopeia. The WHO provides the INN to the Organization's Member States (at present 191), to national pharmacopoeia commissions, and to other bodies designated by WHO’s Member States.
The name, nelitolimod, is ready for use in labelling and publications. It will serve to identify SD-101 during its lifetime worldwide. Going forward, TriSalus will use the name in publications and public statements, at conferences and other forums, and in corporate-related materials.
About TriSalus Life Sciences
TriSalus Life Sciences is an oncology company integrating novel delivery technology with immunotherapy to transform treatment for patients with liver and pancreatic tumors.
The Company’s platform includes devices that utilize a proprietary drug delivery technology and a clinical-stage investigational immunotherapy. The Company’s two FDA-cleared devices use its proprietary Pressure-Enabled Drug Delivery™ (PEDD™) approach to deliver a range of therapeutics: the TriNav
®
Infusion System for hepatic arterial infusion of liver tumors and the Pancreatic Retrograde Venous Infusion System for pancreatic tumors. PEDD is a novel delivery approach designed to address the anatomic limitations of arterial infusion to the pancreas. The PEDD approach modulates pressure and flow in a manner that delivers more therapeutic to the tumor and is designed to reduce undesired delivery to normal tissue, bringing the potential to improve patient outcomes. SD-101, the Company’s investigational immunotherapeutic candidate, is designed to improve patient outcomes by treating the immunosuppressive environment created by many tumors, which can make current immunotherapies ineffective in the liver and pancreas. Patient data generated during Pressure-Enabled Regional Immuno-Oncology™ (PERIO) clinical trials support the hypothesis that SD-101 delivered via PEDD may have favorable immune effects within the liver and systemically. The target for SD-101, TLR9, is expressed across cancer types and the mechanical barriers addressed by PEDD are commonly present as well. SD-101 delivered by PEDD will be studied across several indications in an effort to address immune dysfunction and overcome drug delivery barriers in the liver and pancreas.
In partnership with leading cancer centers across the country – and by leveraging deep immuno-oncology expertise and inventive technology development – TriSalus is committed to advancing innovation that improves outcomes for patients. Learn more at
trisaluslifesci.com
and follow us on
X (formerly Twitter)
and
LinkedIn
.
免疫疗法临床研究上市批准
2024-03-06
LOS ANGELES, March 06, 2024 (GLOBE NEWSWIRE) -- ACTG, a global clinical trials network focused on HIV and other infectious diseases, today made the oral presentation “HepB-CpG Vaccine is Superior to HepB-alum in People with HIV and Prior Vaccine Non-Response (A5379)” at the Conference on Retroviruses and Opportunistic Infections (CROI 2024) in Denver, Colorado. These data demonstrated that the HepB-CpG vaccine achieved up to 99 percent protection among people living with HIV who had previously not responded to conventional hepatitis B vaccines, a noteworthy increase compared to the protection achieved by conventional vaccines. People living with HIV, especially those with lower CD4 counts, often do not develop protective antibodies after receiving conventional hepatitis B vaccines. The HepB-CpG (HEPLISAV-B®) vaccine includes a TLR-9 agonist adjuvant (CpG 1018® adjuvant) and is known to achieve high protection against hepatitis B among people living with HIV, but until now there have been limited data about its protection among people living with HIV who have not responded to conventional hepatitis B vaccines. “Hepatitis B remains a significant issue for people living with HIV, as having both viruses increases the likelihood of liver complications,” said ACTG Chair Judith Currier, M.D., M.Sc., University of California Los Angeles. “Today’s presentation demonstrated extremely high rates of protection among those receiving the HepB-CpG vaccine and are likely to change clinical practice.” A5379 is an ongoing, open-label study that compares protection of the HepB-CpG vaccine to the conventional hepatitis B vaccine among people living with HIV who had not responded to prior hepatitis B vaccination and are on antiretroviral therapy (ART). Of the 561 eligible participants enrolled at 41 sites in 10 countries, 64 percent were male, 42 percent were Black, 35 percent were White, 17 percent were Asian, 22 percent were Hispanic, and the median age was 46 years old. They were equally randomized to one of three groups, receiving either: Two doses of HepB-CpG intramuscularly at week 0 and week 4Three doses of HepB-CpG intramuscularly at week 0, week 4, and week 24Three doses of HepB-alum (conventional hepatitis B vaccine) intramuscularly at week 0, week 4, and week 24 Today’s analysis showed that 93 percent of participants receiving two doses of the HepB-CpG vaccine and 99 percent receiving three doses of the HepB-CpG vaccine achieved protection against hepatitis B, compared to 81 percent receiving three doses of the conventional hepatitis B vaccine. The most frequently reported adverse events were vaccination site pain, fatigue, headache, malaise, and myalgia. No unexpected safety issues were observed. “In all of our HIV clinics, we see large numbers of people living with HIV who have been vaccinated against hepatitis B but show no evidence of a vaccine response,” said Lead Author and Protocol Co-Chair Kristen Marks, M.D., Weill Cornell Medicine. “Today’s remarkable findings are game changing for those individuals and offer them a potential path toward protection against hepatitis B.” A5379 is led by Kenneth Sherman, M.D., Ph.D., University of Cincinnati and Massachusetts General Hospital (Protocol Chair) and Dr. Marks. ACTG is led by Dr. Currier and Joseph J. Eron, M.D., University of North Carolina (ACTG Vice-Chair). It is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (which also funds ACTG) under award numbers UM1 AI068636, UM1 AI107716, and UM1 AI068634. HEPLISAV-B® was provided by Dynavax Technologies Corporation. About ACTGACTG is the world’s largest and longest running clinical trials network focused on HIV and other infectious diseases and the people living with them. It is funded by NIAID and collaborating NIH Institutes. Founded in 1987, ACTG conducts research to improve the management of HIV and its comorbidities; develop a cure for HIV; and innovate treatments for tuberculosis, hepatitis B, and emerging infectious diseases. It comprises thousands of dedicated researchers, staff, and community members who are pursuing research into novel treatments and cures for infectious diseases at 65 locations across four continents, with the ultimate goal of advancing science that meaningfully impacts the lives of the people we serve. Disclaimer: This content is solely the responsibility of ACTG and does not necessarily represent the official views of the NIH. Media Contact:Rachel Reiss, ACTGRLReiss@mednet.ucla.edu
临床结果疫苗临床研究
2022-07-06
The presented data show ELI-005 elicits strong and long-lasting cellular and humoral immune responses that were maintained at significantly higher levels than comparator vaccines over 32 weeks in mice. ELI-005, containing the lymph node-targeted Amphiphile vaccine adjuvant AMP-CpG, induced potent, comprehensive and persistent innate immune responses in draining lymph nodes in mice. Animals treated with AMP-CpG showed a significantly higher percentage of CD8+ T cells with central memory phenotype, which resulted in more robust recall responses one week after antigen challenge in mice. ELI-005 rapidly induced potent cellular and antibody responses in non-human primates up to 5,000-fold over baseline which exhibited cross-reactive neutralization activity specific to Beta, Delta and Omicron SARS-CoV-2 variants of concern. BOSTON, July 06, 2022 (GLOBE NEWSWIRE) -- Elicio Therapeutics, a clinical-stage biotechnology company developing a pipeline of novel immunotherapies for the treatment of cancer and other diseases, today announced the presentation of preclinical data on ELI-005, a protein subunit vaccine containing the spike receptor-binding domain (RBD) protein of SARS-CoV-2, and Elicio’s lymph node-targeted CpG TLR-9 agonist, Amphiphile-CpG (AMP-CpG), demonstrating they induce potent cross-reactive antibody and T cell responses in mice and non-human primates. ELI-005 yielded robust and durable cross-reactive serum IgG responses specific to several SARS-CoV-2 variants of concern alongside potent and cross-reactive peripheral T cell responses. The data were presented in three posters at the 2022 Keystone Symposia on Viral Immunity: Basic Mechanisms and Therapeutic Applications virtually and in-person at the Keystone Resort in Keystone, CO from June 29-July 2, 2022. The electronic presentations are accessible here. While currently authorized vaccines have shown success in the reduction of severe disease risk from COVID-19, rapidly emerging viral variants continue to drive substantial pandemic waves of infection, resulting in numerous global public health challenges. Future advances in prophylactic vaccine activity will play a critical role in the resolution of the current pandemic and preparation for future pandemics driven by similar coronavirus pathogens. The robust immune responses generated by ELI-005 suggest that it may enhance broad protection against new variants of concern. “We are pleased to present updated data from ongoing preclinical work in mice and non-human primates with ELI-005, our lymph node-targeting AMP-vaccine against SARS-CoV-2. The truly exciting component of the data is that we are seeing an immune response that is very broad in its ability to recognize variants of concern that did not exist at the point when we were developing the approach. This potential to address many variants is critically important for any future coronavirus outbreaks,” said Peter DeMuth, Ph.D., Chief Scientific Officer at Elicio. “Overall, ELI-005 generated robust and durable responses from the two arms of adaptive immunity—cellular and humoral. These parallel responses are critical to providing optimal immune protection against pathogens like SARS-CoV-2 and support the differentiation of ELI-005 from existing COVID-19 vaccines.” Christopher Haqq, MD, Ph.D., Executive Vice President, Head of Research and Development and Chief Medical Officer added, “These data sets validate our ability to deliver therapeutic payloads with the Amphiphile platform, driven by our Amphiphile modified TLR-9 agonist ELI-004, directly into the lymph nodes, the ‘schoolhouse of the immune system.’ This approach allows us to educate the T cells in the lymph nodes to recognize the antigen and develop persistent, broad immune specificity for a pathogen.” Poster Presentation Highlights Title: A lymph node targeted protein subunit vaccine induces robust cellular and humoral immunity to SARS-CoV-2 in non-human primates Highlights from the Presentation:
ELI-005, containing the Amphiphile vaccine adjuvant AMP-CpG and WH-01 Spike receptor binding domain (RBD), induced polyfunctional antigen-specific CD8+ and CD4+ T cells in the peripheral blood of non-human primates.ELI-005 rapidly induced potent antibody responses in non-human primates up to 5,000-fold over baseline (reaching 104 binding antibody units/mL) which were cross-reactive to Beta, Delta and Omicron SARS-CoV-2 variants of concern.ELI-005 vaccination with AMP-CpG was safe and well tolerated with no observation of local reactogenicity, or abnormal changes in blood chemistry, hematocrit, body weight, body temperature or serum cytokine levels following each immunization. Title: A lymph node targeted protein subunit vaccine generates strong cellular and cross-reactive humoral immunity against SARS-CoV-2 with potent long-term recall responses Highlights from the Presentation: ELI-005 elicits strong and long-lasting cellular and humoral immune responses. Acute immune responses to ELI-005, one week after boost immunization, resulted in >60% of circulating and lung-resident CD8+ T cells, as well as nearly 10% of lung-resident CD4+ T cells to be reactive to RBD.Over 32 weeks, these responses were maintained at significantly higher levels than comparator vaccines, with 6% of circulating CD8+ T cells having maintained a cytokine+ status at 32 weeks.Animals treated with ELI-005 showed a significantly higher percentage of CD8+ T cells with central memory phenotype, which resulted in more robust recall responses one week after antigen challenge.Strong antibody responses, that were cross-reactive towards RBD variants, were generated and maintained throughout the course of the study. Title: A lymph node targeted CpG, TLR-9 agonist induces potent and persistent activation of lymphatic innate immune responses resulting in robust cellular and humoral immunity to SARS-CoV-2 Highlights from the Presentation:
ELI-005, containing the lymph node-targeted Amphiphile vaccine adjuvant AMP-CpG, induced potent, comprehensive and persistent innate immune responses in draining lymph nodes.AMP-CpG induced increased numbers of all innate cell lineages in the lymph nodes, as well as increased numbers of innate cells expressing co-stimulatory molecules and producing key cytokines.In the lymph nodes, AMP-CpG upregulated numerous pro-inflammatory immune mediators associated with multiple axes of immune activation, including hematopoietic growth factors, cytokines and chemokines. About ELI-004 ELI-004 (AMP-CpG) is an Amphiphile modified TLR-9 agonist that is included in ELI-005. ELI-004 “hitchhikes” on endogenous albumin until it reaches antigen presenting cells in the draining lymph nodes to potently stimulate immune activation and support expansion of adaptive immune responses. ELI-004 has previously demonstrated at least 10-fold improved lymph node delivery, leading to substantially enhanced immune cell delivery and immune responses versus conventional CpG and other benchmark adjuvants. ELI-004 has demonstrated eradication and cures in multiple preclinical cancer models and activity against infectious diseases and, in this study, COVID-19. When the IND was approved for ELI-002 last year, IND approval for ELI-004 was automatically gained as well as it is 1 of 8 components in the asset. About the Amphiphile Platform Our proprietary Amphiphile, or AMP, platform delivers investigational immunotherapeutics directly to the “brain center” of the immune system – the lymph nodes. We believe this site-specific delivery of disease-specific antigens, adjuvants and other immunomodulators may efficiently educate, activate and amplify critical immune cells, potentially resulting in induction and persistence of potent adaptive immunity required to treat many diseases. In preclinical models, we have observed lymph node-specific engagement driving therapeutic immune responses of increased magnitude, function and durability. We believe our AMP lymph node-targeted approach will produce superior clinical benefits compared to immunotherapies that do not engage the lymph nodes.
Our AMP platform, originally developed at the Massachusetts Institute of Technology, or MIT, has broad potential across cancers, infectious diseases and other disease indications to advance a number of development initiatives through internal activities, in-licensing arrangements or development collaborations and partnerships.
The Amphiphile platform has been shown to deliver immunotherapeutics directly to the lymph nodes by latching on to the protein albumin, found in the bloodstream, as it travels to lymphatic tissue. In preclinical models, we have observed lymph node-specific engagement driving therapeutic immune responses of increased magnitude, function and durability.
About Elicio Therapeutics
Elicio Therapeutics is a clinical-stage biotechnology company developing a pipeline of novel immunotherapies for the treatment of cancer and other diseases. By combining expertise in immunology and immunotherapy, Elicio is engineering investigational Amphiphile immunotherapies that are intended to precisely target and fully engage the lymph nodes, the site in our bodies where the immune response is orchestrated. Elicio is engineering lymph node-targeted AMPlifiers, immunomodulators, adjuvants and vaccines for an array of aggressive cancers and infectious diseases.Elicio began dosing subjects in AMPLIFY-201, its Phase 1/2 clinical trial in solid tumor subjects for its lead Amphiphile vaccine, ELI-002, targeting KRAS-driven cancers in October 2021. The Amphiphile platform emerged from the laboratories of Darrell Irvine, Howard Hughes Investigator and Professor of Biomedical Engineering in the Koch Institute of Integrative Cancer Research at MIT. For more information, please visit https://elicio.com/.
Cautionary Note on Forward-Looking Statements
This press release includes forward-looking statements. Such forward-looking statements involve known and unknown risks, uncertainties, assumptions and other important factors that could cause our actual results, performance or achievements to differ materially from historical results or any future results, performance or achievements expressed, suggested or implied by such forward-looking statements. Forward-looking statements include, but are not limited to, statements regarding or expectations for our lymph node-targeted approach to treating cancer and infectious diseases, our interpretation that the strong T cell responses generated by ELI-005 could enhance broad protection against new variants of COVID-19 and the general ability and potential of our proprietary Amphiphile, or AMP, platform, to deliver investigational immunotherapeutics directly to the lymph nodes, including across indications, our expectation that advances in prophylactic vaccine activity will play a critical role in the resolution of the current pandemic and preparation for future pandemics driven by similar coronavirus pathogens. Applicable risks and uncertainties that could cause our actual results, performance or achievements to differ materially from historical results or any future results, performance or achievements expressed, suggested or implied by our forward-looking statements include, among others: the potential that we experience slower than expected enrollment in our clinical trials, we identify serious side effects or other safety issues, we do not have clinical supply of our product candidate that is adequate in amount and quality and supplied in a timely fashion, and the inherent risks of clinical development; the results of our clinical trials do not continue to support our approach and expectation of lymph node targeting for the enhanced treatment of cancer and infectious diseases or that the results do not continue to support that the AMP platform enhances TCR-T clinical responses in solid tumors; our limited operating history and historical losses; our need to raise capital to fund our research and development programs; the early stage nature of the development of our product candidates; our ability to obtain orphan drug designation from the FDA; competition from various competitors in the markets targeted by our product candidates, including from competitors with substantially greater resources than us; our general dependence on third parties in connection with manufacturing, clinical trials and preclinical studies; the potential complexity of the manufacturing process for our product candidates; our ability to protect our intellectual property; our dependence on the patents we license from the Massachusetts Institute of Technology, or MIT; our compliance with healthcare laws and regulations; and risks relating to the impact on of COVID-19 or other infectious diseases on our business. The forward-looking statements contained in this press release reflect our current views with respect to future events, and we do not undertake and specifically disclaim any obligation to update any forward-looking statements, except as required by law.
Media Contact
Gloria Gasaatura LifeSci Communications +1 646-970-4688 ggasaatura@lifescicomms.com
疫苗合作抗体孤儿药免疫疗法
100 项与 Napa9-SPG 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 肿瘤 | 临床前 | 美国 | - |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
