1
项与 Umbilical Cord Derived Mesenchymal Stromal Cell(LiveKidney.Bio) 相关的临床试验A Phase I, Open-Label Study to Evaluate the Safety and Tolerability of Subcutaneous Administration of Umbilical Cord Derived - Mesenchymal Stromal Cell Therapy in Addition to Standard of Care As a Treatment for Active Systemic Lupus Erythematosus
The goal of this clinical trial is to evaluate the safety and effectiveness of UC-MSCs in adults with systemic lupus erythematosus (SLE).
The main questions this study aims to answer are:
1. Can UC-MSCs improve kidney function and reduce SLE disease activity?
2. Are UC-MSCs safe and well-tolerated in this patient population?
Participants in this study will:
* Receive UC-MSCs in a single dose in addition to standard of care treatment.
* Provide blood and urine samples for laboratory assessments, including biomarkers and immune profiling (e.g., cytokines, complement proteins, and autoantibodies).
* Attend regular clinic visits for physical exams, disease activity scoring, and imaging tests to monitor kidney health.
* Complete assessments for safety, such as monitoring for adverse events and changes in laboratory values.
This study aims to provide new insights into treatment options for SLE and lupus nephritis, addressing an unmet medical need in this population.
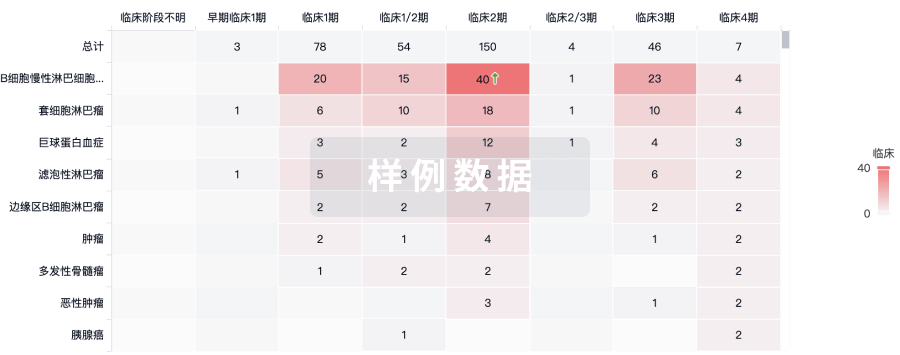
100 项与 Umbilical Cord Derived Mesenchymal Stromal Cell(LiveKidney.Bio) 相关的临床结果
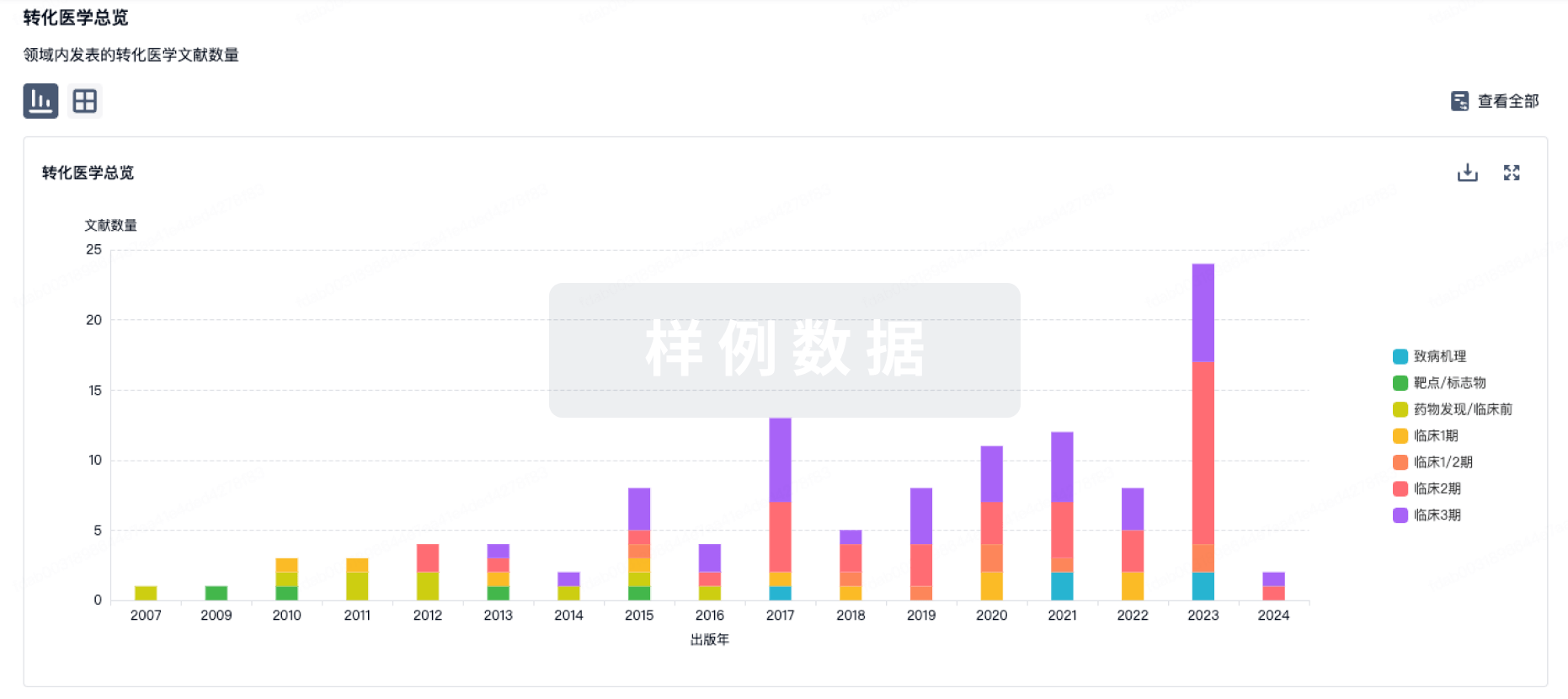
100 项与 Umbilical Cord Derived Mesenchymal Stromal Cell(LiveKidney.Bio) 相关的转化医学
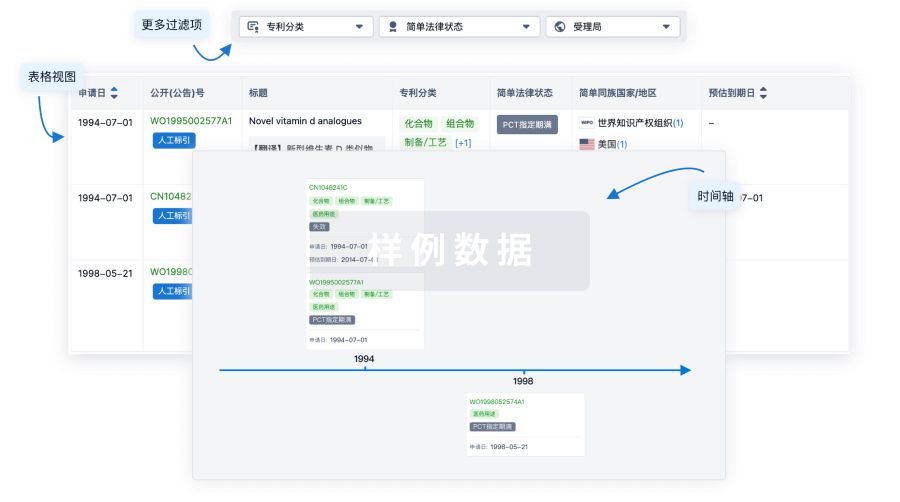
100 项与 Umbilical Cord Derived Mesenchymal Stromal Cell(LiveKidney.Bio) 相关的专利(医药)
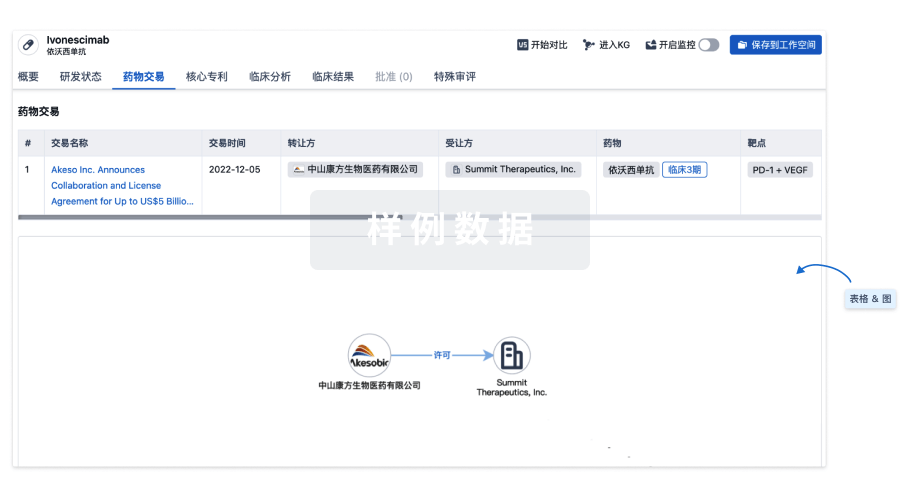
100 项与 Umbilical Cord Derived Mesenchymal Stromal Cell(LiveKidney.Bio) 相关的药物交易