预约演示
更新于:2025-07-19
ARX-305
重组人源化抗CD70单抗-AS269偶联物(Ambrx)
更新于:2025-07-19
概要
基本信息
原研机构 |
非在研机构- |
最高研发阶段临床1期 |
首次获批日期- |
最高研发阶段(中国)临床1期 |
特殊审评- |
登录后查看时间轴
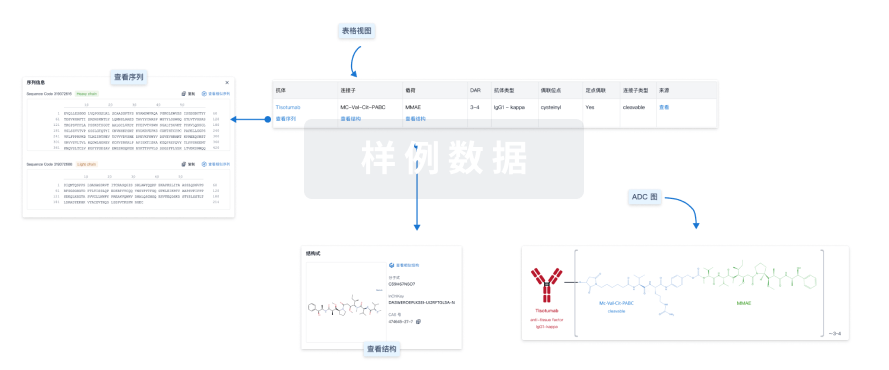
结构/序列
使用我们的ADC技术数据为新药研发加速。
登录
或
关联
1
项与 重组人源化抗CD70单抗-AS269偶联物(Ambrx) 相关的临床试验CTR20222161
评价注射用重组人源化抗CD70单抗-AS269偶联物(ARX305)在晚期肿瘤患者中的安全性、耐受性、药代动力学特征及初步有效性的开放、多中心、I期临床研究
主要目的:评价ARX305的安全性和耐受性;确定ARX305的最大耐受剂量(MTD)和Ⅱ期研究的推荐剂量(PR2D)。
次要目的:评价ARX305及其代谢产物的药代动力学特征;评价ARX305的初步抗肿瘤疗效;评估ARX305的免疫原性;评估CD70表达与ARX305疗效相关性。
开始日期2022-10-19 |
申办/合作机构 |
100 项与 重组人源化抗CD70单抗-AS269偶联物(Ambrx) 相关的临床结果
登录后查看更多信息
100 项与 重组人源化抗CD70单抗-AS269偶联物(Ambrx) 相关的转化医学
登录后查看更多信息
100 项与 重组人源化抗CD70单抗-AS269偶联物(Ambrx) 相关的专利(医药)
登录后查看更多信息
110
项与 重组人源化抗CD70单抗-AS269偶联物(Ambrx) 相关的文献(医药)2023-04-06·Bioconjugate chemistry
One-Pot Assembly of Dual-Site-Specific Antibody-Drug Conjugates via Glycan Remodeling and Affinity-Directed Traceless Conjugation.
Article
作者: Jiang, Zhong-Xing ; Yao, Xu ; Tang, Feng ; Zeng, Yue ; Tang, Caihong ; Zhang, Jianxin ; Huang, Wei ; Zheng, Xing ; Shi, Wei
The drug-to-antibody ratio (DAR) value and dual-drug combination greatly influence the therapeutic index of antibody-drug conjugates (ADCs). The reported approaches usually require multifunctional branched linkers, a combination of complicated technologies, or protein-protein ligation, which may incorporate multihydrophobic fragments or result in low coupling efficiency. Herein, we developed a facile and efficient one-pot method to assemble dual-site-specific ADCs with defined DARs at both the N-glycosylation site and K248 site, either with the same payloads or with two types of payloads. The constructed dual-site ADCs showed acceptable homogeneity, excellent buffer stability, and enhanced in vitro and in vivo efficiency.
2023-03-21·Leukemia & lymphoma
Hepatic Veno-occlusive disease (VOD) in multiple myeloma patient receiving Belantamab Mafodotin
Letter
作者: Trajce, Enkeleida ; Danglis, Fotios ; Argyrakopoulou, Georgia ; Pessach, Ilias ; Chandrinou, Eleni ; Rapti, Irene ; Petropoulos, Fotios
This is a case of a 74 yr old man with a history of IgGkappa MM from 2009.He had undergone treatment with Bortezomib-Dexamethasone at diagnosis, which was repeated 6 years afterwards followed by ASCT and Lenalidomide maintenance.Belamaf is a first-in-class humanized IgG1 ADC targeting B-cell maturation antigen (BCMA), covalently linked via a cysteine linker to the microtubule inhibitor monomethyl auristatin F (MMAF), which is released after internalization of the ADC to BCMA on tumor plasma cell, and inhibits its polymerization with the arrest of G2/M caspase dependent apoptosis.The patient received Levofloxacin 12-wk prophylaxis, acyclovir and co-trimoxazole prophylaxis, hydromellose eye drops throughout the treatment period, a coolant eye mask during every Belamaf infusion, and was ophthalmologicaly assessed before each cycle.2 Wk later, patient was urgently re-admitted with consciousness disturbances, weight gain, abdominal pain, defecation incapacity, and significant hyperbilirubinemia (total bilirubin: 6 mg/dL, direct bilirubin: 3 mg/dL, indirect bilirubin: 3 mg/dL).Our patient had never received an Allo-SCT, he had undergone an Autologous SCT many years before the VOD presentation and he had received several lines of treatment without presenting signs of hepatic toxicity.In conclusion, we described a rare case of VOD, histol. proven, in a MM patient on Belamaf-a novel ADC approved for treatment of relapsed or refractory MM.
2023-03-01·Journal of controlled release : official journal of the Controlled Release Society
Comparison of HER2-targeted affibody conjugates loaded with auristatin- and maytansine-derived drugs
Article
作者: Tretyakova, Maria S ; Tolmachev, Vladimir ; Zhang, Jie ; Belousov, Mikhail V ; Orlova, Anna ; Liu, Yongsheng ; Oroujeni, Maryam ; Yin, Wen ; Ding, Haozhong ; Gräslund, Torbjörn ; Bodenko, Vitalina ; Xu, Tianqi ; Vorobyeva, Anzhelika
Treatment with antibody drug conjugates targeting receptors over-expressed on cancer cells is well established for clinical use in several types of cancer, however, resistance often occurs motivating the development of novel drugs. We have recently investigated a drug conjugate consisting of an affibody molecule targeting the human epidermal growth factor receptor 2 (HER2), fused to an albumin-binding domain (ABD) for half-life extension, loaded with the cytotoxic maytansine derivative DM1. In this study, we investigated the impact of the cytotoxic payload on binding properties, cytotoxicity and biodistribution by comparing DM1 with the auristatins MMAE and MMAF, as part of the drug conjugate. All constructs had specific and high affinity binding to HER2, human and mouse albumins with values in the low- to sub-nM range. ZHER2-ABD-mcMMAF demonstrated the most potent cytotoxic effect on several HER2-over-expressing cell lines. In an experimental therapy study, the MMAF-based conjugate provided complete tumor regression in 50% of BALB/c nu/nu mice bearing HER2-over-expressing SKOV3 tumors at a 2.9 mg/kg dose, while the same dose of ZHER2-ABD-mcDM1 provided only a moderate anti-tumor effect. A comparison with the non-targeting ZTaq-ABD-mcMMAF control demonstrated HER2-targeting specificity. In conclusion, a combination of potent cytotoxicity in vitro, with minimal uptake in normal organs in vivo, and efficient delivery to tumors provided a superior anti-tumor effect of ZHER2-ABD-mcMMAF, while maintaining a favorable toxicity profile with no observed adverse effects.
46
项与 重组人源化抗CD70单抗-AS269偶联物(Ambrx) 相关的新闻(医药)2025-06-28
·药事纵横
声明:因水平有限,错误不可避免,或有些信息非最及时,欢迎留言指出。本文仅作医疗健康相关药物介绍,非治疗方案推荐(若涉及);本文不构成任何投资建议。在医药行业的赛道上,新和成(002001)与浙江医药(600216)这对“同省兄弟”一直备受关注,两家均以维生素类营养品为主业,但是发展路径、拓展方向却又不同,接下来从业绩、主营构成、研发、人效薪酬、发展战略等维度拆解对比,看谁才是浙江医药板块的领跑者。 业绩增长对比2024年度,新和成以216.1亿的总营收占据领先位置,其营收同比增长42.95%,净利润58.69亿元,同比增长117.01%。2024年公司香精香料板块实现营收39.16亿元,同比+19.62%,新材料板块实现营收16.76亿元,同比+39.51%。浙江医药2024年总营收93.75亿元,同比增长20.29%,净利润39.68亿元,净利润同比增幅57.21%,表现依然可圈可点,尤其值得关注的是其净利润率高达42%。从2020-2024年营收复合增长率来看,新和成达到20.31%,远高于浙江医药的6.36%;净利润复合增长来看,新和成的13.33%和浙江医药的12.82%,旗鼓相当。充分体现了,新和成除了维生素,布局氨基酸、香精香料、新材料大赛道的做大营收规模的明智之举;图:新和成主营收入构成情况浙江医药主营构成为维生素、社会配送及商业和药品,净利复合增长近13%,对成本管控也是优秀。图:浙江医药主营产品构成内稳外拓,殊途同归2024年度浙江医药国内外营收占比2:1,其国内营收62.34亿元,占67.6%;国外营收31.03亿元,收入占32.4%。新和成则与之相反,国内仅95.56亿元,占比44.2%,国外营收120.53亿元,占比达55.8%。两家企业的营收占比情况呈现出明显的"内需主导型"和"外向驱动型"结构。浙江医药战略定位“内需筑基,渐进出海”,国内营收占比2成,在国内以医保市场为主,奈诺沙星等创新药深度绑定国内临床需求,原料药托底。而国际营收主攻高端市场,维生素系列以欧美为主,2024年新增创新药海外临床三期项目3个。而新和成国际营收占比呈现大宗产品+长协订单的特征,营养品(维生素A/D3)出口占比高并且新和成的国际收入规模接近浙江医药的4倍,展现出其全球化布局的领先优势。营养品板块,蛋氨基酸项目产能得到释放,实现30万吨/年产能。从营收国内外布局情况,我们可以看出,两家企业都在积极布局海外市场,稳固国内市场,以实现营收利润的稳步增长。客户结构对比从客户结构来看,浙江医药Top5客户销售额14.08亿,占总销售额的15.02%,而新和成Top5客户销售额共24.45亿元,占总销售额比例仅11.31%。浙江医药精准聚焦,15%的集中度反映其创新药推广特性,依赖国内头部流通商快速打开医保市场,不过浙江医药战略布局中也在平衡创新药放量与渠道控制力。新和成隐形分散,11%的低集中度,国际订单虽然分散,但是核心品类实际被DSM/巴斯夫等巨头的长协订单锁定。新和成的第一大客户为其提供了11.29亿元的销售额,占年度总销售额的5.23%。研发投入,企业创新的核心引擎研发投入一直是一家企业突破核心技术的关键所在。浙江医药2024年度研发费用投入7.49亿元,研发费用同比下滑12.87%,研发费用占总营收的7.99%。据其财报披露,主要由于公司部分研发项目于2024年度进入收尾阶段,相应研发费用支出减少所致。而新和成2024年度总研发投入10.36亿元,研发费用同比增长16.71%,研发费用占比略低于浙江医药,仅4.79%。但新和成也连续多年研发投入占比营业收入超过5%,搭建了从基础研究、工程化开发、工艺流程优化到产品应用开发的创新研发体系,促进产业的转型升级。截至报告期末,浙江医药共有在研新药新产品项目4项,处于临床研究或BE阶段6项,申报生产10项,仿制药在研20项。浙江医药创新药稳步推进,搭建自主技术平台。一类新药奈诺沙星胶囊及注射剂相继在国内获批上市,与其他喹诺酮类药物相比,奈诺沙屋具备突出的药效和安全性优势,有望成为未来喹诺酮类抗生素领域的重要力量。在ADC创新药管线上,浙江医药与AMBRX Inc.合作开发ARX788与ARX305,并构建了基于非天然氨基酸定点偶联生物大分子药物自主技术平台,可广泛应用于生物大分子药物的改造。截至2023年,ARX788临床I期研究已经达到期中分析预设的有效性标准,ARX305正在进行临床一期试验,两种药剂未来市场前景广阔。并于2017年投资浙江新码生物,开始布局ADC管线,以下为新码生物的研发管线。图:浙江新码生物研发管线(浙江医药控股子公司)下面我们结合两家企业近两年的研发人员数量变化和研发人员占比情况一起来看一下:从整体数据来看,新和成的研发人员数量和占比均高于浙江医药,2024年新和成研发人员数量2867人,对比2023年增加了64人,研发人员占比从23.85%上升至25.22%,表明其产业转型升级中以研究院作为企业技术产业核心的战略正在逐步实施。而浙江医药2024年度研发人员数量对比2023年有所下降,从866人到760人到调整,研发人员占比也从13.91%下降至13.07%。研发是企业的发展命脉,既决定当下技术护城河的深度也可能拓宽未来赛道的广度,新和成生物法降本重塑行业的格局,浙江医药押注ADC冲击未来市场,差异化的研发投入折射出不同企业的战略局部和未来发展方向。人均创收、创利及人均薪酬对比人均创收和创利以及人均薪酬对比情况一直是大家非常关注的企业运营指标。从浙江医药和新和成的以下三项数据的对比,我们不难看出,新和成的人均创收190.07万元,略高于浙江医药的161.22万元,但是人均创利角度来看,新和成人均创利51.62万元,是浙江医药19.96万元的2.5倍多,结合两者,我们可以看出虽然人均创收相近的情况,但是新和成的人均创利水平更胜一筹。员工人均薪酬方面来看,新和成平均薪酬18.47万元略高于浙江医药的17.02万元的平均薪酬。结合两家企业的研发人员数量情况,我们不难看出两家企业的战略布局方向的差异,新和成营养品/香精香料高度标准化,30万吨蛋氨酸产能自动化率高,人员成本相对较低,而浙江医药创新药研发+制剂生产依赖高端人力,临床团队也更依赖高端人才。备注:2024年度人均薪酬/人均创收/人均创利数据来自wind;人均薪酬算法:((应付职工薪酬合计:期末余额-劳务派遣薪酬合计:期末余额)-(应付职工薪酬合计:期初余额劳务派遣薪酬合计:期初余额)+(支付给职工以及为职工支付的现金-劳务派遣薪酬合计:本期增加))/((期初员工总数+期未员工总数)/2);人均创收算法:营业总收入/员工人数;人均创利算法:归属母公司股东的净利润/员工人数发展战略新和成:立足于精细化工行业,以“化工+”、“生物+”为核心技术平台,围绕营养品、香精香料、新材料、原料药生产各种功能性化学品。加强技术平台和产业平台的建设,加强先进装备的引进和合作,依托浙江新昌、浙江上虞、山东潍坊、黑龙江绥化等四个现代化生产基地,实现产业链的延伸,推动公司的可持续高质量发展,同时积极关注并培育植保产业、新能源产业、节能环保产业和信息产业等战略新兴产业相关的功能化学品机遇。浙江医药:坚定立足药物、药物制剂以及药品市场,理清思路、端正方向、落实措施。同时,利用现有的生命营养产品、保健食品生产线及营销基础优势,将大健康产品作为重点发展的方向,并不断开拓新的领域。总的来说,新和成通过灵活开放激进的策略,快速布局氨基酸,香精香料,新材料这些大赛道,营收规模上的很快。浙江医药立足医药,采用稳步推进的策略,将大健康产品作为重点发展的方向。参考资料:www.cnhugroup.comwww.zmc.top
财报临床3期
2025-05-03
·赛柏蓝
编者按:本文来自米内网,作者苍穹;赛柏蓝授权转载,编辑相宜踏入5月,上市公司2024年业绩基本披露完毕,年度最会赚钱的医药企业名单也随之出炉。截至4月30日,2024年净利润超过10亿元的A股医药企业共有46家,合计净利润在1200亿元以上;其中,迈瑞、恒瑞、云南白药、上药等6家企业均以超40亿元的净利润位居前列;达仁堂、浙江医药、百利天恒等6家企业净利润增长均超过100%;22家企业净利润创新高,包括华润三九、华东等首度突破30亿大关的潜力者,以及国药现代、西藏药业等首次晋身10亿梯次的生力军。2024年净利润超过10亿元的A股医药企业01医疗药械龙头116亿净利润出圈76亿分红创新高作为国产医疗药械龙头,迈瑞医疗2024年实现营业收入367.26亿元,同比增长5.14%;净利润116.68亿元,同比增长0.74%。自公司上市以来,迈瑞医疗营收和净利润已保持连续7年的双增长。分红预案显示,迈瑞医疗拟向全体股东每10股派5.6元(含税),共计派发现金红利约6.79亿元。加上2024年已实施的中期利润分配和第二次中期利润分配,2024年度公司累计现金分红总额将达到76.02亿元,现金分红比例为65.15%,分红总金额再创历史新高。过去的2024年,其已完成“设备+IT+AI”的智能医疗生态系统搭建,推出全球首个临床落地的重症医疗大模型——启元重症医疗大模型,并通过“三瑞”生态与设备的融合创新,结合大数据、人工智能,为医疗机构提供全院级数智化整体解决方案,用技术手段解决优质医疗资源分布不均的痛点,助力全球医疗机构提升诊疗能力和运营效率,同时提高其在全球高端客户群的渗透率和品牌粘性。对于国际市场局部面临宏观环境和地缘政治带来的挑战,迈瑞医疗表示,得益于海外高端客户群的持续突破、本地化平台能力建设的逐步完善,以及全球各主要地区收入的均衡分布,特别是在发展中国家和欧洲市场强劲表现的拉动下,其国际业务收入有望在未来几年内不断提升。02净利润涨超47%恒瑞医药创新成果丰硕素有“创新药一哥”之称的恒瑞医药,2024年实现营业收入279.85亿元,同比增22.63%;净利润63.37亿元,同比增47.28%;拟向全体股东按每10股派发现金股利2.00元(含税),为自上市以来第25次派现分红。近年来,恒瑞医药创新成果持续涌现,临床价值凸显,驱动收入增长。2024年创新药销售收入达138.92亿元(含税,不含对外许可收入),同比增长30.60%,创新药销售收入占总销售收入(不含对外许可收入)一半以上,研发投入占销售收入比重达29.40%,至今恒瑞累计研发投入已超过460亿元。为保证创新产出,恒瑞不断加大创新力度,2024年研发投入达82.28亿元,其中费用化研发投入65.83亿元,截至目前,已有约20款1类新药在国内获批上市。BD国际化加速推进,恒瑞已累计实现15笔创新药海外授权合作,其中近三年合计对外授权项目数占比过半,包括多笔总交易金额在10亿美元以上的项目。03云南白药净赚47亿核心单品销量节节攀升云南白药2024年实现营业收入400.33亿元,同比增长2.36%;净利润47.49亿元,同比增长16.02%,业绩再现双增。基于良好的业绩表现及发展质效,云南白药持续提升股东回报水平,2024年度拟派发现金红利21.14亿元,2024年三季度已实施的特别分红21.64亿元,以上现金分红累计总额达42.79亿元,占全年净利润的90.09%。2024年,云南白药进一步聚焦工业板块。药品事业群方面,销售额过亿的产品达10个,其中云南白药气雾剂大涨26%,蒲地蓝消炎片、气血康口服液分别涨超22%和14%,云南白药膏、云南白药胶囊等均实现显著增长;中药资源事业群持续保障战略品种的供应与价格稳定,三七提取物销售继续保持市场领先水平;健康品事业群净利润同比增长8.36%,口腔护理及防脱洗护领域稳健发展。云南白药将创新研发作为高质量发展的底层支撑:布局气血康口服液、云南白药胶囊等11个中药大品种、25个项目的二次创新开发;推进云南白药透皮制剂、全三七片、附杞固本膏等创新中药开发;聚焦创新药研发成果转化,如INR101(前列腺癌患者PSMA阳性病灶PET成像)、INR102(转移性去势抵抗性前列腺癌)已分别步入Ⅲ期和Ⅰ/Ⅱa期临床,KA-1641(肿瘤恶病质)处于临床前研究阶段。04华润三九净利润首破30亿中药传承创新成绩斐然华润三九2024年交出了一份营收利润双增、且均创新高的答卷,公司实现营业收入276.17亿元,同比增长11.63%;净利润33.68亿元,同比增长18.05%。公司拟向全体股东每10股派送现金3.2元(含税),预计派发现金红利4.11亿元。在复杂的经济和市场环境下,华润三九2024年继续锚定战略方向,坚持“品牌+创新”双轮驱动。自我诊疗(CHC)业务成绩亮眼:999感冒灵、抗病毒口服液等大品种市场持续扩大,冰连清咽喷雾剂、玉屏风口服液等新品快速打开市场;中药传承创新成果显著:4个经典名方获批上市,包括首个进入医保目录的妇科经典名方温经汤颗粒;多款新药临床试验稳步推进:1类新药KYAZ01-2011-020(急性脑卒中)、CR999C1905L1(抗肿瘤)、CR999C2016L1(胶质瘤)等已进入Ⅰ期临床及以上阶段,2类改良型新药CR999K1601L1(胃癌根治术淋巴示踪)、CR999C1701L1(痤疮)等已进入Ⅲ期临床。此外,华润三九通过“内生+外延”双向发力,持续完善产业布局。其收购昆药集团后,2024年完成对华润圣火51%股权的收购,强化三七产业链掌控力。目前昆药集团净利润贡献占比达22.3%,其慢病管理业务与公司CHC板块形成协同。随着其对天士力28%股权收购,有望进一步补充其心脑血管创新中药管线。05浙江医药净利润暴增170%子公司表现可圈可点浙江医药2024年实现营业收入93.75亿元,同比增长20.29%;净利润11.61亿元,同比增长170.11%。其拟每10股派发红利3.7元(含税),预计现金分红3.56亿元,占净利润比例为30.66%。作为国内维矿领域的“扛把子”,浙江医药表示,2024年其主导产品维生素E和维生素A市场价格和需求上升,利润进一步增长;生命营养品和药品两大主营业务板块销售实现双涨,推动整体营收增长,进而带动利润提升;优化成本费用结构,研发费用下降12.87%,销售费用维持平稳,使其保持较高的利润水平。浙江医药多个子公司在2024年表现出彩,合力为业绩贡献增量。其中,子公司芳原馨生物通过优化间甲酚各相关中间体生产工艺,综合完工成本下降;子公司新码生物积极推进1类新药ARX788(HER2 ADC)的生产许可证取证工作,ARX305(CD70 ADC)Ⅰ期临床试验正进行中,NCB003(长效IL-2药物)获得临床试验默示许可;子公司新昌制药厂的1类新药SH-337(治疗消化系统酸相关性疾病)、LYSC98(由革兰阳性菌引起的细菌性皮肤和软组织感染)等已相继获批临床。来源:东方财富网、公司年报;注:统计日期截至4月30日,如有疏漏,欢迎指正!END内容沟通:郑瑶(13810174402)
财报
2025-05-03
·米内网
精彩内容踏入5月,上市公司2024年业绩基本披露完毕,年度最会赚钱的医药企业名单也随之出炉。截至4月30日,2024年净利润超过10亿元的A股医药企业共有46家,合计净利润在1200亿元以上;其中,迈瑞、恒瑞、云南白药、上药等6家企业均以超40亿元的净利润位居前列;达仁堂、浙江医药、百利天恒等6家企业净利润增长均超过100%;22家企业净利润创新高,包括华润三九、华东等首度突破30亿大关的潜力者,以及国药现代、西藏药业等首次晋身10亿梯次的生力军。2024年净利润超过10亿元的A股医药企业医疗药械龙头116亿净利润出圈,76亿分红创新高作为国产医疗药械龙头,迈瑞医疗2024年实现营业收入367.26亿元,同比增长5.14%;净利润116.68亿元,同比增长0.74%。自公司上市以来,迈瑞医疗营收和净利润已保持连续7年的双增长。分红预案显示,迈瑞医疗拟向全体股东每10股派5.6元(含税),共计派发现金红利约6.79亿元。加上2024年已实施的中期利润分配和第二次中期利润分配,2024年度公司累计现金分红总额将达到76.02亿元,现金分红比例为65.15%,分红总金额再创历史新高。过去的2024年,公司已完成“设备+IT+AI”的智能医疗生态系统搭建,推出全球首个临床落地的重症医疗大模型——启元重症医疗大模型,并通过“三瑞”生态与设备的融合创新,结合大数据、人工智能,为医疗机构提供全院级数智化整体解决方案,用技术手段解决优质医疗资源分布不均的痛点,助力全球医疗机构提升诊疗能力和运营效率,同时提高公司在全球高端客户群的渗透率和品牌粘性。对于国际市场局部面临宏观环境和地缘政治带来的挑战,迈瑞医疗表示,得益于公司海外高端客户群的持续突破、本地化平台能力建设的逐步完善,以及全球各主要地区收入的均衡分布,特别是在发展中国家和欧洲市场强劲表现的拉动下,公司国际业务收入有望在未来几年内不断提升。净利润涨超47%!恒瑞医药创新成果丰硕素有“创新药一哥”之称的恒瑞医药,2024年实现营业收入279.85亿元,同比增22.63%;净利润63.37亿元,同比增47.28%;拟向全体股东按每10股派发现金股利2.00元(含税),为公司自上市以来第25次派现分红。近年来,恒瑞医药创新成果持续涌现,临床价值凸显,驱动收入增长。2024年公司创新药销售收入达138.92亿元(含税,不含对外许可收入),同比增长30.60%,创新药销售收入占公司总销售收入(不含对外许可收入)一半以上,研发投入占销售收入比重达29.40%,至今恒瑞累计研发投入已超过460亿元。为保证创新产出,恒瑞不断加大创新力度,2024年研发投入达82.28亿元,其中费用化研发投入65.83亿元,截至目前,公司已有约20款1类新药在国内获批上市。BD国际化加速推进,恒瑞已累计实现15笔创新药海外授权合作,其中近三年合计对外授权项目数占比过半,包括多笔总交易金额在10亿美元以上的项目。云南白药净赚47亿,核心单品销量节节攀升云南白药2024年实现营业收入400.33亿元,同比增长2.36%;净利润47.49亿元,同比增长16.02%,业绩再现双增。基于良好的业绩表现及发展质效,云南白药持续提升股东回报水平,2024年度拟派发现金红利21.14亿元,2024年三季度已实施的特别分红21.64亿元,以上现金分红累计总额达42.79亿元,占公司全年净利润的90.09%。2024年,云南白药进一步聚焦工业板块。药品事业群方面,公司销售额过亿的产品达10个,其中云南白药气雾剂大涨26%,蒲地蓝消炎片、气血康口服液分别涨超22%和14%,云南白药膏、云南白药胶囊等均实现显著增长;中药资源事业群持续保障公司战略品种的供应与价格稳定,三七提取物销售继续保持市场领先水平;健康品事业群净利润同比增长8.36%,口腔护理及防脱洗护领域稳健发展。云南白药将创新研发作为公司高质量发展的底层支撑:布局气血康口服液、云南白药胶囊等11个中药大品种、25个项目的二次创新开发;推进云南白药透皮制剂、全三七片、附杞固本膏等创新中药开发;聚焦创新药研发成果转化,如INR101(前列腺癌患者PSMA阳性病灶PET成像)、INR102(转移性去势抵抗性前列腺癌)已分别步入Ⅲ期和Ⅰ/Ⅱa期临床,KA-1641(肿瘤恶病质)处于临床前研究阶段。华润三九净利润首破30亿,中药传承创新成绩斐然华润三九2024年交出了一份营收利润双增、且均创新高的答卷,公司实现营业收入276.17亿元,同比增长11.63%;净利润33.68亿元,同比增长18.05%。公司拟向全体股东每10股派送现金3.2元(含税),预计派发现金红利4.11亿元。在复杂的经济和市场环境下,华润三九2024年继续锚定战略方向,坚持“品牌+创新”双轮驱动。公司自我诊疗(CHC)业务成绩亮眼:999感冒灵、抗病毒口服液等大品种市场持续扩大,冰连清咽喷雾剂、玉屏风口服液等新品快速打开市场;中药传承创新成果显著:4个经典名方获批上市,包括首个进入医保目录的妇科经典名方温经汤颗粒;多款新药临床试验稳步推进:1类新药KYAZ01-2011-020(急性脑卒中)、CR999C1905L1(抗肿瘤)、CR999C2016L1(胶质瘤)等已进入Ⅰ期临床及以上阶段,2类改良型新药CR999K1601L1(胃癌根治术淋巴示踪)、CR999C1701L1(痤疮)等已进入Ⅲ期临床。此外,华润三九通过“内生+外延”双向发力,持续完善产业布局。公司收购昆药集团后,2024年完成对华润圣火51%股权的收购,强化三七产业链掌控力。目前昆药集团净利润贡献占比达22.3%,其慢病管理业务与公司CHC板块形成协同。随着公司对天士力28%股权收购,有望进一步补充其心脑血管创新中药管线。浙江医药净利润暴增170%,子公司表现可圈可点浙江医药2024年实现营业收入93.75亿元,同比增长20.29%;净利润11.61亿元,同比增长170.11%。公司拟每10股派发红利3.7元(含税),预计现金分红3.56亿元,占净利润比例为30.66%。作为国内维矿领域的“扛把子”,浙江医药表示,2024年其主导产品维生素E和维生素A市场价格和需求上升,公司利润进一步增长;生命营养品和药品两大主营业务板块销售实现双涨,推动公司整体营收增长,进而带动利润提升;优化成本费用结构,研发费用下降12.87%,销售费用维持平稳,使公司保持较高的利润水平。浙江医药多个子公司在2024年表现出彩,合力为公司业绩贡献增量。其中,子公司芳原馨生物通过优化间甲酚各相关中间体生产工艺,综合完工成本下降;子公司新码生物积极推进1类新药ARX788(HER2 ADC)的生产许可证取证工作,ARX305(CD70 ADC)Ⅰ期临床试验正进行中,NCB003(长效IL-2药物)获得临床试验默示许可;子公司新昌制药厂的1类新药SH-337(治疗消化系统酸相关性疾病)、LYSC98(由革兰阳性菌引起的细菌性皮肤和软组织感染)等已相继获批临床。来源:东方财富网、公司年报注:统计日期截至4月30日,如有疏漏,欢迎指正!免责声明:本文仅作医药信息传播分享,并不构成投资或决策建议。本文为原创稿件,转载文章或引用数据请注明来源和作者,否则将追究侵权责任。投稿及报料请发邮件到872470254@qq.com稿件要求详询米内微信首页菜单栏商务及内容合作可联系QQ:412539092【分享、点赞、在看】点一点不失联哦
财报
100 项与 重组人源化抗CD70单抗-AS269偶联物(Ambrx) 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 晚期癌症 | 临床1期 | 中国 | 2022-10-19 | |
| CD70阳性肿瘤 | 临床1期 | 中国 | 2022-10-19 | |
| 肿瘤 | 临床申请批准 | 中国 | 2022-07-08 | |
| 血液肿瘤 | 临床申请 | 美国 | 2022-02-15 | |
| 实体瘤 | 临床申请 | 美国 | 2022-02-15 | |
| 多发性骨髓瘤 | 临床前 | 美国 | - |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用


