预约演示
更新于:2025-06-12
BVX-001
更新于:2025-06-12
概要
基本信息
药物类型 ADC |
别名 BVX 100 vcMMAE、BVX-130-mcMMAF、BVX130-mcMMAF + [2] |
作用方式 抑制剂 |
作用机制 CD33抑制剂(髓系细胞表面抗原CD33抑制剂)、CD7抑制剂(T细胞抗原CD7抑制剂)、微管蛋白抑制剂 |
非在研适应症- |
非在研机构- |
权益机构- |
最高研发阶段临床前 |
首次获批日期- |
最高研发阶段(中国)- |
特殊审评孤儿药 (美国) |
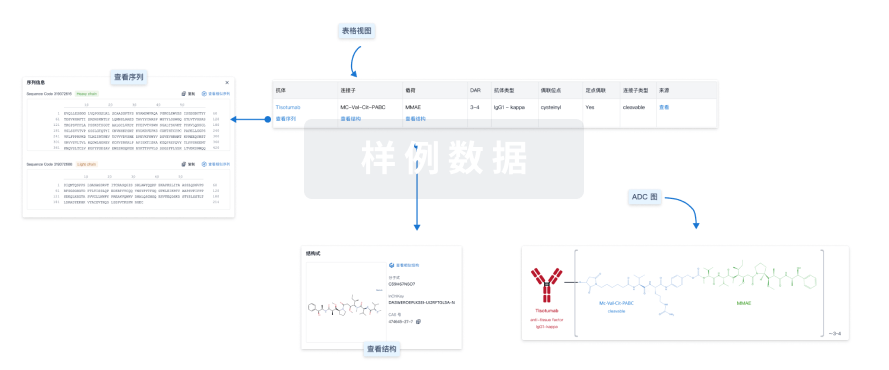
结构/序列
分子式C11H22N4O4 |
InChIKeyAGGWFDNPHKLBBV-YUMQZZPRSA-N |
CAS号159858-33-0 |
查看全部结构式(2)
使用我们的ADC技术数据为新药研发加速。
登录
或
关联
100 项与 BVX-001 相关的临床结果
登录后查看更多信息
100 项与 BVX-001 相关的转化医学
登录后查看更多信息
100 项与 BVX-001 相关的专利(医药)
登录后查看更多信息
586
项与 BVX-001 相关的文献(医药)2025-12-31·mAbs
A bispecific antibody-drug conjugate targeting pCAD and CDH17 has antitumor activity and improved tumor-specificity
作者: Gesner, Thomas ; Logel, Claude ; Xie, Kathleen T. ; Cebe, Regis ; Wu, Nila C. ; Li, Xun ; Shi, Xingyi ; Velazquez, Roberto ; Simmons, Quincey ; Tschantz, William R. ; Barzaghi-Rinaudo, Patrizia ; Korn, Joshua ; Sagar, Vivek ; Hainzl, Dominik ; Malamas, Anthony ; Mercan, Samuele ; Green, Andrew ; McLaughlin, Margaret ; Huber, Thomas ; Mueller, Kathrin ; D’Alessio, Joseph A. ; Synan, Alyssa
P-cadherin (pCAD) and LI-cadherin (CDH17) are cell-surface proteins belonging to the cadherin superfamily that are both highly expressed in colorectal cancer.This co-expression profile presents a novel and attractive opportunity for a dual targeting approach using an antibody-drug conjugate (ADC).In this study, we used a unique avidity-driven in vitro screening approach to generate pCAD x CDH17 bispecific antibodies that selectively target cells expressing both antigens over cells expressing only pCAD or only CDH17.Based on in vitro binding and inhibition of cell proliferation results, we selected a lead bispecific antibody to link to the cytotoxic payload monomethyl auristatin E (MMAE) to generate a pCAD x CDH17 bispecific MMAE ADC.In in vivo dual flank mouse models, we demonstrated antitumor activity of the bispecific ADC in tumors expressing both antigens but not in tumors expressing only pCAD or only CDH17.Overall, the preclin. data presented here support the proof-of-concept bispecific antibody discovery approach, demonstrating a rational design for screening antibodies by prioritizing cross-arm avid IgGs to target dual-pos. cells.
2025-05-21·BIOCONJUGATE CHEMISTRY
Easy Access to Bioorthogonal Click-to-Release Reagent Bishydroxy-trans-cyclooctene (C2TCO) and Harnessing of Its Rapid Labeling and Dissecting Feature in Multicycle Imaging
Article
作者: Kim, Wook ; Yoo, Tae Hyeon ; Kim, Eunha ; Arun, V. ; Choi, Junwon ; Lee, Sangwoo ; Choi, Hongseo ; Lee, Minju
Bifunctional trans-cyclooctene (bTCO) with a carbamate or carbonate at the allylic position and tetrazine provide a promising bioorthogonal click chemistry pair for the click-to-release approach, successfully employed in various biotechnological applications. Herein, we demonstrate a simple and straightforward method to synthesize C2TCO, a symmetrical bTCO derivative with two hydroxyl groups at the allylic positions. The efficiently synthesized C2TCO at first was selectively functionalized with a fluorophore (C2TCO-FL), and the conjugate was labeled onto monoclonal antibodies (Ab-C2TCO-FL). The fluorophore of Ab-C2TCO-FL was easily removed from the antibody through the mild treatment of tetrazine, enabling multicycle fluorescent bioimaging. Next, an antibody-drug conjugate targeting PD-L1 was prepared using the linker based on C2TCO. The cytotoxic payload was efficiently released from the antibody upon tetrazine treatment, which induced cellular cytotoxicity.
2025-05-21·BIOCONJUGATE CHEMISTRY
Elucidating Critical Factors of Internalization and Drug Release of Antibody-Drug Conjugates (ADCs) Using Kinetic Parameters Evaluated by a Novel Tool Named TORCH
Article
作者: Hannewald, Jens ; Shan, Min ; Deutsch, Carl ; Hecht, Stefan ; Sweeney-Lasch, Stanley ; Kolmar, Harald ; Rasche, Nicolas ; Quillmann, Marie ; Anderl, Jan ; Piater, Birgit ; Dickgiesser, Stephan
During the past decade, antibody-drug conjugates (ADCs) have emerged as new drugs in cancer therapy with 15 ADCs already approved such as Kadcyla, Enhertu, and Adcetris. ADCs contain a cytotoxic drug that is linked to an antibody, allowing for specific delivery of the warhead to tumor cells. Typically, the antibody targets a tumor-specific antigen expressed on the cell surface. After the internalization of ADCs into cells, the linker is often cleaved by enzymes in the lysosomal compartment of the cell, releasing the warhead and thereby allowing for its interaction with, for example, the DNA or the tubulin cytoskeleton, which finally leads to cell death. Consequently, binding, internalization, and drug release are key attributes for the efficacy of ADCs. Here, we describe a novel molecule named TORCH (Turn On after Release by CatHepsins) that contains a fluorescence quencher system that is separated by a cathepsin B-cleavable linker. When conjugated to an antibody, the TORCH molecule allows one to gain valuable insights on the internalization and drug release of ADCs. While we cannot exclude the influence of other factors such as receptor recycling, we have found that the receptor density is directly related to the amount of payload released intracellularly, meaning that the internalization per receptor is very similar for all investigated antibodies and cell lines.
13
项与 BVX-001 相关的新闻(医药)2025-02-20
·抗体圈
首款获批的抗体偶联药物(ADC)吉妥珠单抗奥唑米星,曾为癌症治疗带来新希望。但由于患者出现严重副作用甚至死亡病例,该药物随后被加上黑框警告并撤市。不过,这并未阻止生物制药公司在该领域加快研究,以期开发出更安全的替代药物。在研发耐受性良好的癌症疗法的竞赛中,BiVictriX 公司在开发急性髓系白血病(AML)靶向治疗药物方面处于领先地位。
急性髓系白血病是一种血癌,据估计,仅在美国,今年就有超过 2 万人受其影响。它约占所有癌症的 1%,起源于未成熟的白细胞。由于这些白细胞未完全发育成熟,不具备保护身体免受感染的特性。随着这些细胞不受控制地增多,红细胞和血小板的数量会下降,从而引发白血病的一些症状,如疲劳、感染风险增加、皮肤易瘀伤和关节疼痛。和其他癌症一样,急性髓系白血病的确切病因尚不完全清楚,但辐射暴露增加、被诊断出患有某些血液疾病以及之前接受过癌症治疗,都会增加患急性髓系白血病的风险。
目前,化疗、干细胞移植以及放射治疗被视为标准治疗方案,但抗体偶联药物如今正重新兴起。抗体偶联药物由单克隆抗体、连接子和抗癌药物组成,单克隆抗体能与癌细胞中的癌蛋白结合,连接子则在抗体与癌细胞结合后输送抗癌药物。因此,与传统化疗不同,这类药物往往能避开健康细胞,是一种更具针对性的癌症治疗方法。目前,已有 14 种抗体偶联药物上市,其中不少是在过去几年获批的。
BiVictriX 公司位于英国柴郡,其针对急性髓系白血病的主打项目是 BVX001,这是一种双特异性抗体偶联药物,靶向 CD7 和 CD33 两种抗原的组合。这些抗原存在于白血病细胞上,近四分之一的急性髓系白血病患者体内都有。
1.BiVictriX 如何解决抗体偶联药物的缺陷?BiVictriX 首席执行官蒂芙尼・索恩表示:“我们设计这款药物,是为了保留抗体偶联药物的所有强效优势,同时提高对癌细胞的选择性。急性髓系白血病最大的问题之一是严重的毒性,尤其是对骨髓的毒性,会导致中性粒细胞减少和中性粒细胞减少性败血症,这实际上是致命的。事实上,它是急性髓系白血病患者的主要死因之一。我们的主打治疗药物 BVX001,对靶向癌细胞非常有效,但在目前的测试中,似乎不会对骨髓产生任何毒性。”
化疗常见的副作用是骨髓毒性,当骨髓产生的免疫细胞减少时就会出现。这可能导致中性粒细胞减少,即对抗感染的中性粒细胞数量下降,使身体更容易受到感染;还可能引发中性粒细胞减少性败血症,这是中性粒细胞减少的并发症,可能致命。
索恩表示,在临床前研究中,BiVictriX 取得了令人期待的结果,如果这些结果在临床试验中得以重现,那将具有革命性意义。在带有人类急性髓系白血病细胞系的小鼠模型帮助下,小鼠背部长出实体瘤。然后给它们注射候选药物 BVX001,在第一种模型中,肿瘤缩小了 93%。在第二种模型中,肿瘤生长程度更大,但在 28 天结束时,观察到肿瘤大小减少了 97%。
索恩说:“一开始,我们选择这个极具挑战性的细胞系,确实让研究难度增加了不少。但我们是有意为之,就是为了充分展示这款药物的优势。” 她还补充说,这些结果 “在统计学上具有高度显著性”。
2.靶向治疗的前景除了临床前试验,该药物在今年早些时候还进行了安全性研究。BVX001 在人源化小鼠模型上进行测试,这种模型复制了人类免疫系统。该研究将 BVX001 与吉妥珠单抗奥唑米星(商品名 Mylotarg)进行对比,吉妥珠单抗奥唑米星在 2010 年撤市,由于急性髓系白血病研究缺乏重大突破,2017 年它以较低剂量重新获批。研究显示,BVX001 不会导致骨髓毒性或中性粒细胞减少,证明其安全性良好。
索恩说:“据我们所知,没有其他人在研究这个特定概念。我们设计的抗体偶联药物能够真正区分癌细胞和健康细胞。基本上,这种药物只与癌细胞结合,并在癌细胞内释放有效载荷。”
这样可以确保有效载荷不会在血浆或可能表达相似抗原的健康细胞中释放。索恩表示,这款药物专为 60 岁及以上不适合强化治疗的患者设计,对于那些需要一种没有市场上许多其他药物副作用的疗法的患者来说,它可能是一种有效且能治愈疾病的选择。
索恩说:“如果临床前数据能在临床中得到验证,我认为这将是一款非常重要的药物,特别是对于那些无法承受其他强化治疗的患者。目前急性髓系白血病市场上的大多数药物都是小分子药物,它们针对导致疾病的特定分子机制。而作为一家研发抗体偶联药物的公司,我们主要针对癌细胞,将一种非常有效的化疗药物输送到癌细胞内部,从内部将其杀死。”
此外,由于该药物靶向白血病细胞表面的两种抗原,这些抗原就像细胞的特定标签,只有当这些抗原存在时,药物才会被细胞内化,从而使健康细胞免受损害。
这项技术的灵感源于索恩在英国国家医疗服务体系(NHS)接受临床免疫学家培训期间。索恩说:“我想正是那段经历让我有了独特的见解…… 如果你观察癌细胞表面的模式,会发现它们实际上有这些‘条形码’,这使你能够在样本中区分癌细胞和健康细胞。”2017 年,索恩在获得初始投资后创立了 BiVictriX,以进一步推进其药物研发管线。
3.该平台能治疗多种癌症吗?由于 BVX001 的作用机制可用于治疗其他类型的癌症,该公司的 Bi-Cygni ADC 平台在开发另外两种候选药物 BVX002 和 BVX003 时也发挥了关键作用,这两种药物用于治疗卵巢癌、膀胱癌和实体瘤。目前,这两种候选药物都处于研发阶段,很快将进入临床前试验。
为了展示 BVX001 对急性髓系白血病的临床疗效,BiVictriX 希望最终能通过合作推进抗体偶联药物的开发。本月,该公司通过有条件募资筹集了 200 万英镑(约 258 万美元),距离将其抗体偶联药物技术推向市场又近了一步。
“我真的非常相信这项技术,主要是因为它长期以来一直用于诊断目的。在英国、欧洲和美国,这项技术已经用于诊断某些类型的癌症长达 20 多年。我只是很惊讶,居然没有人从治疗角度尝试利用它来减少临床副作用。希望我们的临床前数据能在临床中得到良好验证,我们能够生产出没有目前常见严重副作用的药物。”
参考来源:This biotech is defying leukemia with promising targeted therapies
内容来源于网络,如有侵权,请联系删除。
放射疗法抗体药物偶联物
2024-09-09
This announcement contains inside information for the purposes of Article 7 of Regulation (EU) No 596/2014 as it forms part of UK domestic law by virtue of the European Union (Withdrawal) Act 2018. With the publication of this announcement via a Regulatory Information Service, this inside information is now considered to be in the public domain.
NOT FOR PUBLICATION OR RELEASE IN OR INTO THE UNITED STATES OR AUSTRALIA, CANADA, JAPAN, NEW ZEALAND, THE REPUBLIC OF IRELAND OR THE REPUBLIC OF SOUTH AFRICA, OR ANY PROVINCE OR TERRITORY THEREOF OR TO OR FOR THE ACCOUNT OF ANY NATIONAL, RESIDENT OR CITIZEN OF THE UNITED STATES OR ANY PERSON RESIDENT IN AUSTRALIA, CANADA, JAPAN, NEW ZEALAND, THE REPUBLIC OF IRELAND OR THE REPUBLIC OF SOUTH AFRICA.
BIVICTRIX THERAPEUTICS PLC (“BiVictriX” or the “Company”)
First in vivo data shared from BVX002, BiVictriX’s lead solid tumour bispecific ADC programme Complete tumour growth inhibition and tumour regressions observed at well-tolerated doses in a murine model of ovarian cancer
First in vivo data shared from BVX002, BiVictriX’s lead solid tumour bispecific ADC programme Complete tumour growth inhibition and tumour regressions observed at well-tolerated doses in a murine model of ovarian cancer
First in vivo data shared from BVX002, BiVictriX’s lead solid tumour bispecific ADC programme Complete tumour growth inhibition and tumour regressions observed at well-tolerated doses in a murine model of ovarian cancer
First in vivo data shared from BVX002, BiVictriX’s lead solid tumour bispecific ADC programme Complete tumour growth inhibition and tumour regressions observed at well-tolerated doses in a murine model of ovarian cancer
First in vivo data shared from BVX002, BiVictriX’s lead solid tumour bispecific ADC programme Complete tumour growth inhibition and tumour regressions observed at well-tolerated doses in a murine model of ovarian cancer
First in vivo data shared from BVX002, BiVictriX’s lead solid tumour bispecific ADC programme Complete tumour growth inhibition and tumour regressions observed at well-tolerated doses in a murine model of ovarian cancer
Alderley Park, 9 September 2024 – BiVictriX Therapeutics plc (AIM: BVX), a drug discovery and development company applying an innovative, proprietary approach to develop a new class of highly selective, next generation cancer therapeutics, bispecific antibody drug conjugates (“Bi-Cygni® ADCs”), which exhibit superior potency, whilst reducing treatment-related toxicities, today announces positive initial in vivo data for its lead solid tumour programme, BVX002.
The preclinical study is investigating BVX002 in a murine xenograft model of ovarian cancer, using the human cell line OVCAR-3. Initial data reported highly significant tumour growth inhibitions across all doses tested, ranging from 78.3% to >100% (p-value <0.001). Tumour regressions of up to 63.1% were also observed at the mid and high doses.
During the study, BVX002 was dosed once weekly, with a total of four doses given. All doses of BVX002, including the highest dose tested, were well tolerated in all mice, even with the drug having target cross-reactivity in mice. Following completion of the dosing phase, monitoring of tumour volume post dosing continues. It is noted that the tumours have continued to shrink in the mid to high dose arms two weeks post treatment cessation, highlighting the potential durability of BVX002’s anti-cancer effects. This data builds upon the strong preclinical in vivo efficacy and safety data previously reported on our most advanced bispecific ADC asset, BVX001.
BVX002 targets a novel, cancer-specific antigen pair found to be selectively expressed in a wide range of solid tumour indications, including cases of ovarian cancer and non-small cell lung cancer, but is absent from healthy cells. Evidence from internal data assessing primary patient samples suggests BVX002’s targeted cancer-specific antigen pair may be present in 60-70% of patients with high grade serous ovarian cancer.
In June 2024, BiVictriX announced the awarding of a ca.£0.4 million grant from Innovate UK to help support this preclinical work with BVX002. The Company will use insights from this study, together with additional planned studies, to file an Investigational New Drug (IND) Application with the FDA for BVX002 in due course.
Tiffany Thorn, Chief Executive Officer of BiVictriX Therapeutics plc, commented: “This first in vivo data marks the start of what we anticipate being an incredibly exciting preclinical data package for our lead solid tumour asset, BVX002. We are highly encouraged by the significance of the anti-tumour effect delivered by our therapeutic lead in this hard-to-treat model of ovarian cancer, which showcases the clear benefit of our proprietary bispecific ADC approach in a solid tumour setting. We look forward to providing further updates as we expedite the development of this asset.”
Graph 1: Mean Tumour Volume Over Time for BVX002-treated and Control Groups
Graph 2: Bodyweight Change for BVX002-treated and Control Groups
ENDS
For more information, please contact:
BiVictriX Therapeutics plc Tiffany Thorn, Chief Executive Officer Michael Kauffman, Non‐Executive Chairman Email: info@bivictrix.com
SP Angel Corporate Finance LLP (NOMAD and Broker) David Hignell, Caroline Rowe (Corporate Finance) Vadim Alexandre, Rob Rees (Sales and Broking) Tel: +44 (0) 20 3470 0470
Panmure Liberum Limited (Joint Broker) Emma Earl, Freddy Crossley, Mark Rogers, Rupert Dearden Tel: +44 (0) 20 7886 2500
ICR Consillium Lucy Featherstone, Namrata Taak, Max Bennett, Emmalee Hoppe Tel: +44 (0) 20 3709 5700 Email: bivictrix@consilium-comms.com
About BiVictriX Therapeutics plc
BiVictriX (AIM: BVX) is an emerging biotechnology company leveraging clinical experience and its proprietary discovery engine to advance a new class of highly cancer-selective, next-generation precision cancer therapies in one of the fastest-growing markets in oncology. BiVictriX’s first-in-class Bi-Cygni® Antibody Drug Conjugates (“Bi-Cygni®ADCs”) combine superior efficacy with substantially improved cancer-selectivity and safety to provide opportunities for prolonged dosing and greater efficacy in the clinic. The Company is advancing its pipeline to deliver the future of cancer care across a broad range of haematological and solid cancer indications in areas of high unmet medical need.
Find out more about BiVictriX online at www.bivictrix.com. and connect with us on LinkedIn and Twitter @BiVictriX.
抗体药物偶联物临床结果
2024-08-12
Bivictrix Therapeutics joined AIM almost exactly three years ago, raising 7.5 million pounds sterling ($9.5 million) from an IPO.
Antibody-drug conjugates (ADCs) have been at the center of many a billion-dollar biobuck licensing deal over the last year, but Bivictrix Therapeutics feels like it’s been missing out.The preclinical company—which is currently listed on the U.K.’s ailing AIM stock exchange—is keen to get its lead bispecific candidate BVX001 into human trials, but currently has just 1.7 million pounds sterling ($2.2 million) to its name. After mulling its options, the biotech’s leadership has decided the best way to raise fresh funds is to go private.Bivictrix said it has already had “positive initial interactions” with the FDA about moving BVX001, a CD7xCD33 candidate for acute myeloid leukemia, into the clinic. Now, it requires “significant funds to be able to proceed.”“In comparison to private companies operating in the ADC space, the directors believe the current market capitalisation of the company neither fully reflects the positive achievements nor the underlying prospects of the business and is a barrier to future growth, funding and potential partnership and licensing discussions,” Bivictrix said in an Aug. 12 release.The company name-checked fellow U.K.-based ADC company Myricx Bio, which last month raised 90 million pounds ($114 million) in a series A round to take its own candidates into the clinic as illustrating “the appetite for major investors to invest in this area.” The current levels of liquidity available from trading the company’s shares on AIM “do not, in itself, offer investors the opportunity to trade in meaningful volumes or with frequency within an active market,” Bivictrix explained.“Whilst there is no guarantee that cancellation and re-registration will lead to the company successfully completing a significant fundraise or licensing deal, the directors believe its prospects of such a transaction will be significantly increased as a private company,” Bivictrix said.Bivictrix will be following a well-worn path of biotechs like Destiny Pharma as well as other companies that are fleeing AIM, a junior exchange to the London Stock Exchange, citing a desire to raise money elsewhere or the cost of listing requirements.Bivictrix joined AIM almost exactly three years ago, raising 7.5 million pounds ($9.5 million) from an IPO that saw the company list its shares for 20 pence apiece. The company has lost 35% of its value in the following years, trading at 13 pence on Friday.Shareholders will be asked to vote on the plan to go private at a meeting at the end of the month.

抗体药物偶联物IPO
100 项与 BVX-001 相关的药物交易
登录后查看更多信息
研发状态
10 条进展最快的记录, 后查看更多信息
登录
| 适应症 | 最高研发状态 | 国家/地区 | 公司 | 日期 |
|---|---|---|---|---|
| 骨髓增生异常综合征 | 临床前 | 英国 | 2025-06-01 | |
| 急性髓性白血病 | 临床前 | 英国 | 2020-08-15 |
登录后查看更多信息
临床结果
临床结果
适应症
分期
评价
查看全部结果
| 研究 | 分期 | 人群特征 | 评价人数 | 分组 | 结果 | 评价 | 发布日期 |
|---|
No Data | |||||||
登录后查看更多信息
转化医学
使用我们的转化医学数据加速您的研究。
登录
或

药物交易
使用我们的药物交易数据加速您的研究。
登录
或

核心专利
使用我们的核心专利数据促进您的研究。
登录
或

临床分析
紧跟全球注册中心的最新临床试验。
登录
或

批准
利用最新的监管批准信息加速您的研究。
登录
或

生物类似药
生物类似药在不同国家/地区的竞争态势。请注意临床1/2期并入临床2期,临床2/3期并入临床3期
登录
或

特殊审评
只需点击几下即可了解关键药物信息。
登录
或

Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用
