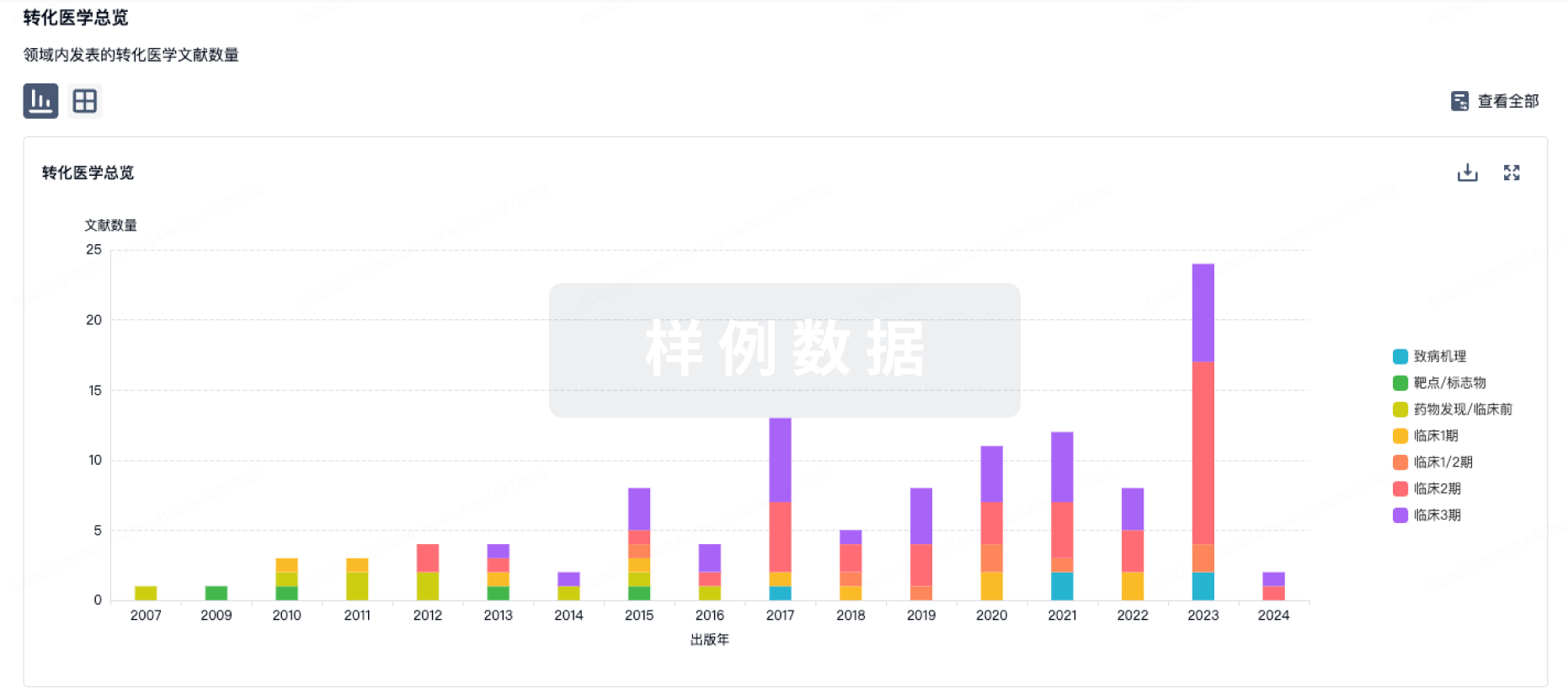
Pathological loss-of-function mutations in cyclin-dependent kinase-like 5 (CDKL5) cause CDKL5 deficiency disorder (CDD), a rare and severe neurodevelopmental disorder associated with severe and medically refractory early-life epilepsy, motor, cognitive, visual, and autonomic disturbances in the absence of any structural brain pathology. Analysis of genetic variants in CDD has indicated that CDKL5 kinase function is central to disease pathology. CDKL5 encodes a serine-threonine kinase with significant homology to GSK3β, which has also been linked to synaptic function. Further, Cdkl5 knock-out rodents have increased GSK3β activity and often increased long-term potentiation (LTP). Thus, development of a specific CDKL5 inhibitor must be careful to exclude cross-talk with GSK3β activity. We synthesized and characterized specific, high-affinity inhibitors of CDKL5 that do not have detectable activity for GSK3β. These compounds are very soluble in water but blood–brain barrier penetration is low. In rat hippocampal brain slices, acute inhibition of CDKL5 selectively reduces postsynaptic function of AMPA-type glutamate receptors in a dose-dependent manner. Acute inhibition of CDKL5 reduces hippocampal LTP. These studies provide new tools and insights into the role of CDKL5 as a newly appreciated key kinase necessary for synaptic plasticity. Comparisons to rodent knock-out studies suggest that compensatory changes have limited the understanding of the roles of CDKL5 in synaptic physiology, plasticity, and human neuropathology.