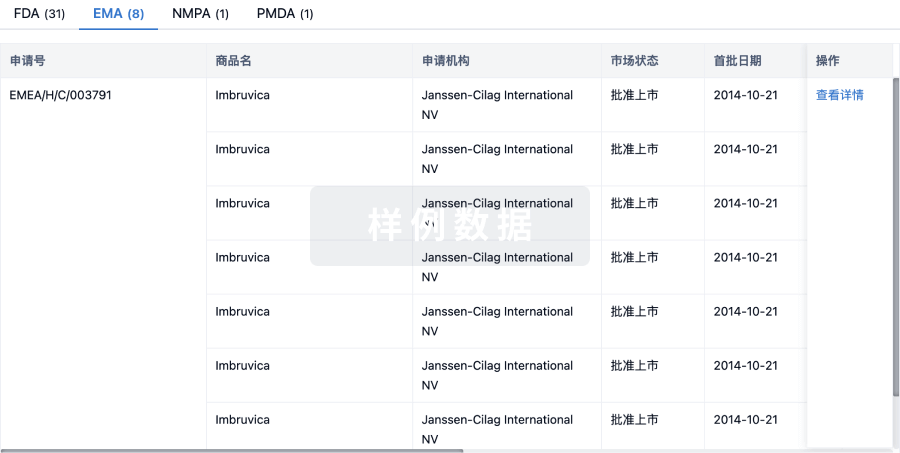
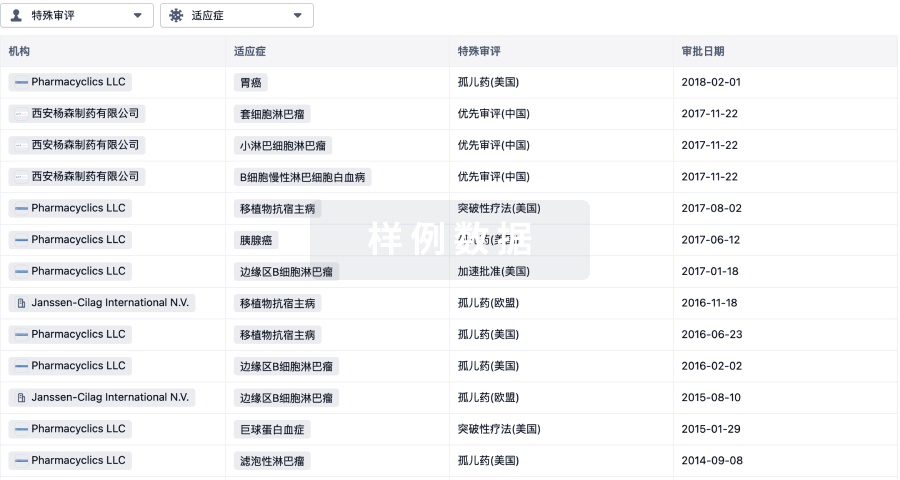
1
项与 CMV-specific HIV-CAR T Cells(City of Hope National Medical Center) 相关的临床试验A Pilot Study to Evaluate the Feasibility and Safety of Cytomegalovirus-Specific, Anti-HIV Chimeric Antigen Receptor (CMV-HIV CAR) T Cells in People Living With HIV
Human immunodeficiency virus type 1 (HIV-1) causes a persistent infection that ultimately leads to acquired immunodeficiency syndrome (AIDS). Treatment of HIV-1 infection with combination anti-retroviral therapy (ART) suppresses HIV-1 replication to undetectable viral levels and saves lives. Nevertheless, ART cannot eradicate latent cellular reservoirs of the virus, and HIV-1 infection remains a life-long battle. Adoptive cellular immunotherapy using chimeric antigen receptor (CAR) engineered T cells directed against HIV-1 envelope subunit protein gp120 (HIVCAR T cells) may provide a safe and effective way to eliminate HIV-infected cells.
However, the number of HIV-infected cells is low in participants under ART, and CAR T cells disappear if they are not stimulated by their target antigens. Interestingly, about 95% of HIV-1-infected individuals are CMV-seropositive and CMV-specific T cells have been shown to persist. To overcome the CAR T cells low persistence issue, we propose to make HIV-CAR T cells using autologous cytomegalovirus (CMV)-specific T cells, which can be stimulated by endogenous CMV in vivo. The overall hypothesis of this first-in-human Phase 1, open-label, single-arm study is that endogenous immune signals to CMV-specific T cells can maintain the presence of autologous bispecific CMV/HIV-CAR T cells in healthy people living with HIV-1 (PLWH), and achieve long-term remission in the presence of ART.
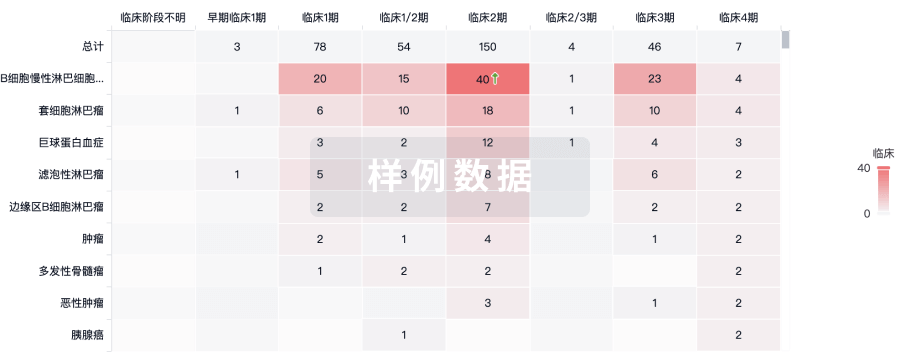
100 项与 CMV-specific HIV-CAR T Cells(City of Hope National Medical Center) 相关的临床结果
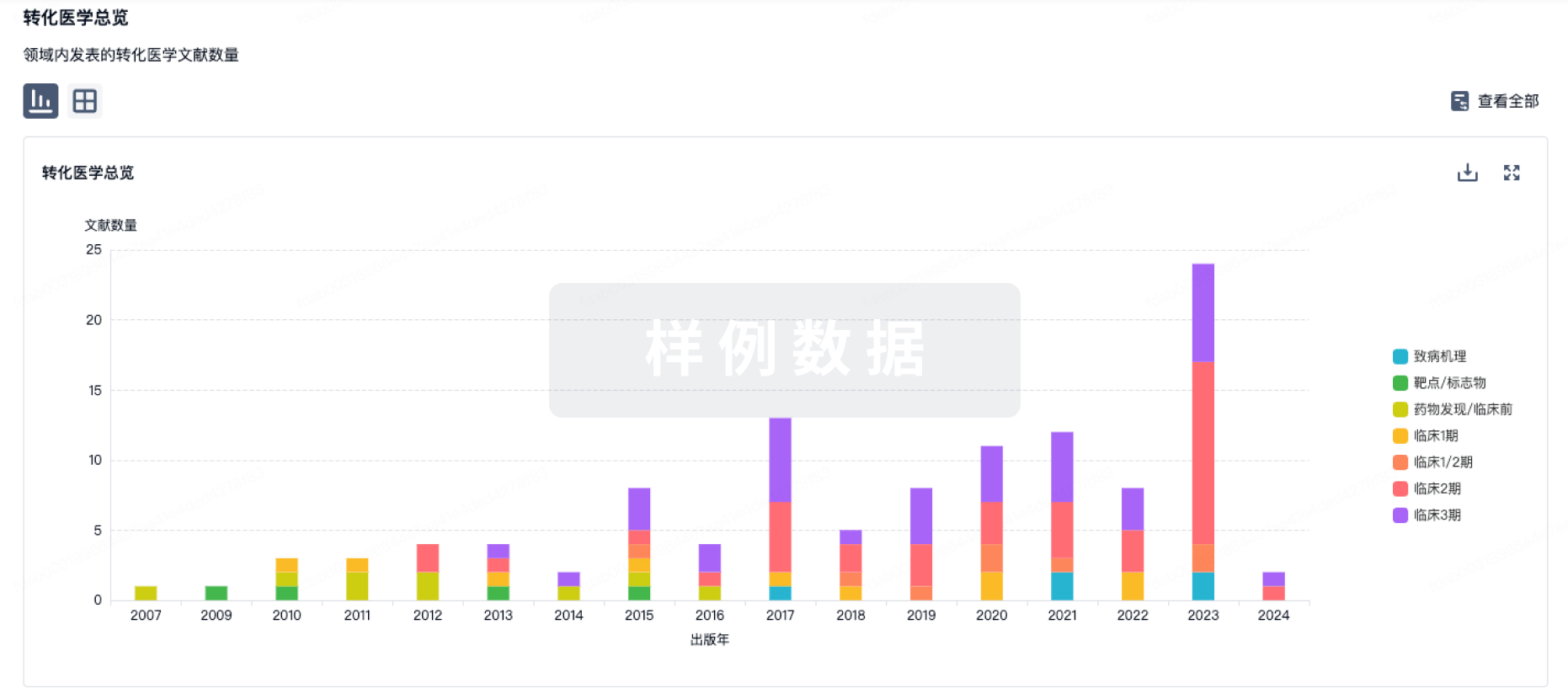
100 项与 CMV-specific HIV-CAR T Cells(City of Hope National Medical Center) 相关的转化医学
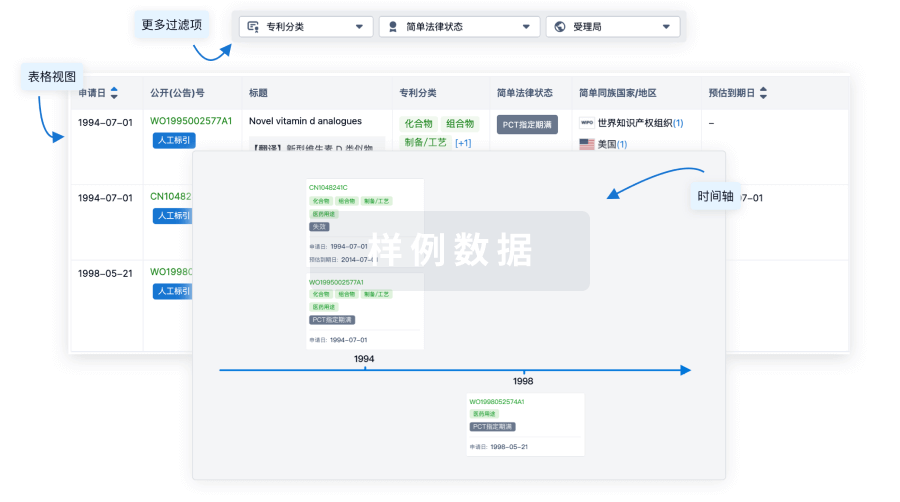
100 项与 CMV-specific HIV-CAR T Cells(City of Hope National Medical Center) 相关的专利(医药)
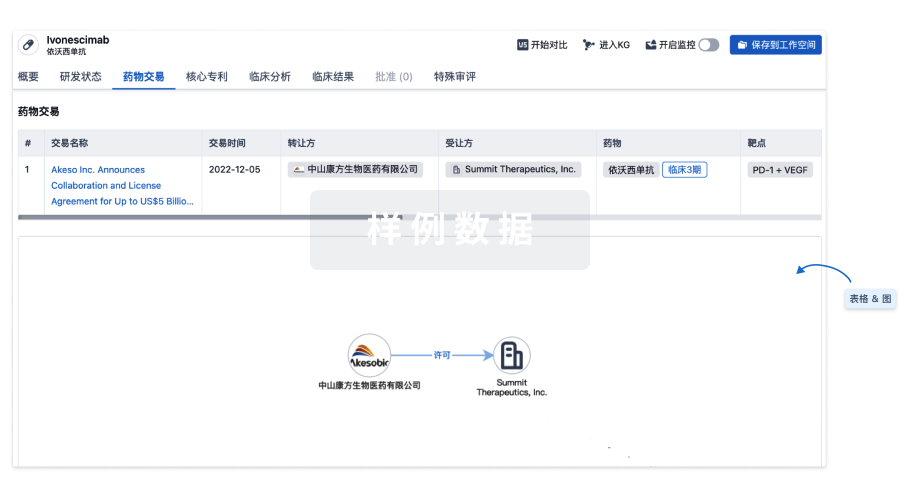
100 项与 CMV-specific HIV-CAR T Cells(City of Hope National Medical Center) 相关的药物交易