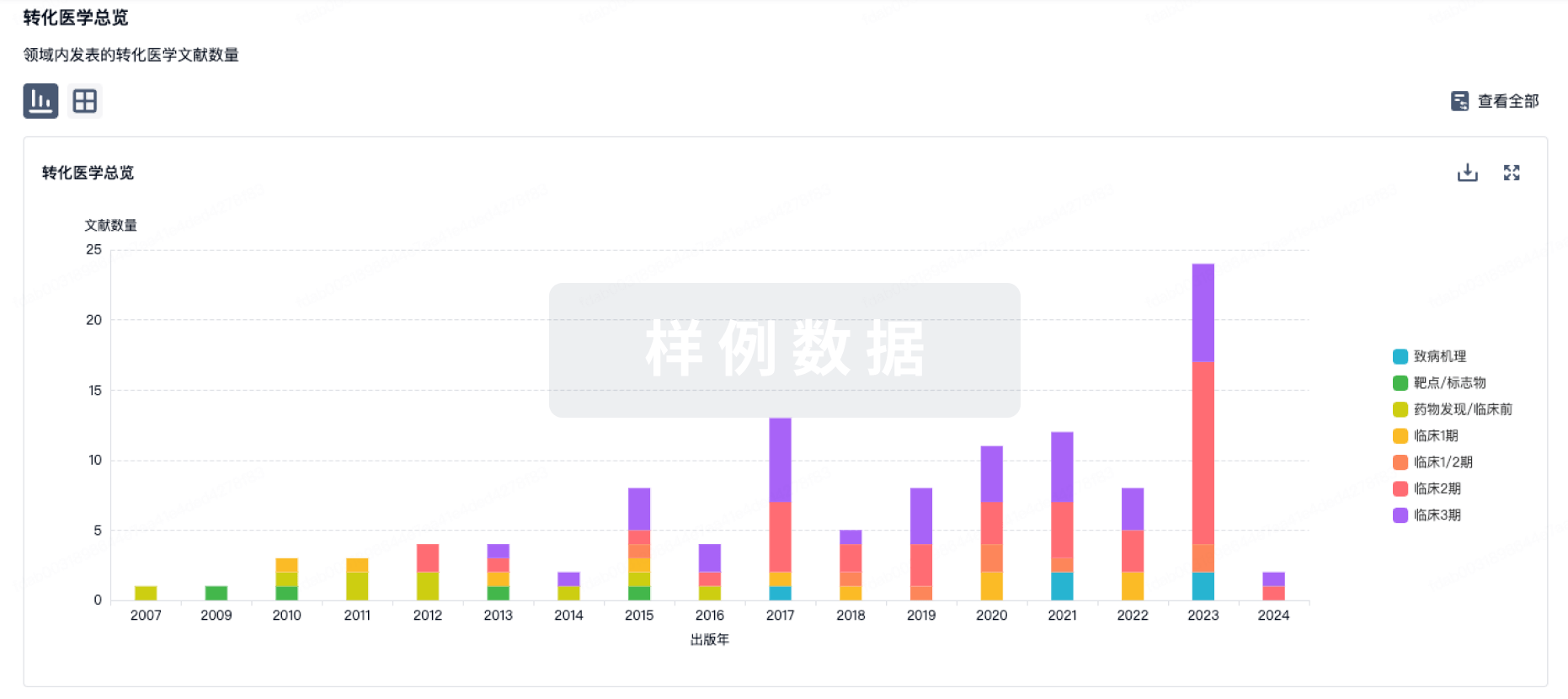
1区 · 医学
Article
作者: Chao, Hsin-Yi ; Lin, Tony Eight ; Lai, Mei-Jung ; Huang, Fang-I ; Li, Yu-Hsuan ; Huang, Hsiang-Ling ; Hsu, Kai-Cheng ; Yeh, Teng-Kuang ; Lee, Hsueh-Yun ; Yang, Chia-Ron ; Liou, Jing-Ping ; Fan, Sheng-Jun
This paper reports the development of a series of 5-aroylindolyl-substituted hydroxamic acids. N-Hydroxy-4-((5-(4-methoxybenzoyl)-1 H-indol-1-yl)methyl)benzamide (6) has potent inhibitory selectivity against histone deacetylase 6 (HDAC6) with an IC50 value of 3.92 nM. It decreases not only the level of phosphorylation of tau proteins but also the aggregation of tau proteins. Compound 6 also shows neuroprotective activity by triggering ubiquitination. In animal models, compound 6 is able to ameliorate the impaired learning and memory, and it crosses the blood-brain barrier after oral administration. Compound 6 can be developed as a potential treatment for Alzheimer's disease in the future.