预约演示
更新于:2025-05-07
Prostatitis
前列腺炎
更新于:2025-05-07
基本信息
别名 Inflammation of prostate、Inflammation of the prostate、Inflammatory disease of prostate, unspecified + [22] |
简介 Infiltration of inflammatory cells into the parenchyma of PROSTATE. The subtypes are classified by their varied laboratory analysis, clinical presentation and response to treatment. |
关联
64
项与 前列腺炎 相关的药物靶点- |
作用机制- |
在研机构 |
原研机构 |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2020-08-27 |
靶点- |
作用机制- |
在研机构 |
原研机构 |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2020-03-19 |
靶点- |
作用机制- |
在研机构 |
原研机构 |
在研适应症 |
非在研适应症- |
最高研发阶段批准上市 |
首次获批国家/地区 中国 |
首次获批日期2020-03-06 |
288
项与 前列腺炎 相关的临床试验NCT06822751
Exploratory Pragmatic Trial of Fosfomycin-trometamol Treatment of Male Urinary Tract Infections in Primary Care
Male urinary tract infections (MUTI) are often less recognised compared to those in women. French clinical guidelines practices recommend the use of antibiotics called fluoroquinolones, which are highly effective in treating MUTIs. However, these antibiotics can lead to rare but serious side effects, such as tendonitis or heart rhythm disturbances. Additionally, fluoroquinolones can contribute to the development of bacterial resistance, making their use inadvisable within six months of treatment.
In response to these concerns, we aim to explore a well-established alternative, fosfomycin trometamol (known by the brand name MONURIL®). This antibiotic has a strong track record in treating UTIs in women, with well-documented benefits and minimal associated risks.
The primary goal of this study is to assess the effectiveness of fosfomycin trometamol in treating urinary tract infections in men, as well as to evaluate any potential treatment failures.
In response to these concerns, we aim to explore a well-established alternative, fosfomycin trometamol (known by the brand name MONURIL®). This antibiotic has a strong track record in treating UTIs in women, with well-documented benefits and minimal associated risks.
The primary goal of this study is to assess the effectiveness of fosfomycin trometamol in treating urinary tract infections in men, as well as to evaluate any potential treatment failures.
开始日期2025-06-01 |
申办/合作机构- |
CTRI/2025/03/083739
A study on management of chronic prostatitis by basti therapy - NIL
开始日期2025-04-25 |
申办/合作机构- |
ITMCTR2025000661
Clinical efficacy and proteomic study of electroacupuncture in type III prostatitis
开始日期2025-04-10 |
申办/合作机构- |
100 项与 前列腺炎 相关的临床结果
登录后查看更多信息
100 项与 前列腺炎 相关的转化医学
登录后查看更多信息
0 项与 前列腺炎 相关的专利(医药)
登录后查看更多信息
7,303
项与 前列腺炎 相关的文献(医药)2025-12-11·The Aging Male
Prostatitis and male infertility
Review
作者: Hua, Lin ; Gao, Xintao ; Zhan, Junfeng ; Wu, Xiaolong ; Liu, Hanchao
2025-09-01·Biomaterials
MXene-loaded multifunctional nanoparticles with on-demand controlled antimicrobial and antioxidant capacity for multi-modal treating bacterial prostatitis
Article
作者: Li, Ruixiao ; Chen, Xi ; Zheng, Yunhe ; Gao, Yanyao ; Wang, Ke ; Wang, He ; Zhang, Yuchen ; Song, Bin ; Liu, Kailai ; He, Jiangchuan ; Zhang, Geng ; Huang, Yu ; Wang, Lei ; Fu, Qiang
2025-07-01·Phytomedicine
Juglone alleviates pelvic pain and prostatic inflammation via inhibiting the activation of NLRP3 inflammasome and alleviating oxidative stress in EAP mice
Article
作者: Zhang, Yi ; Tai, Sheng ; Yue, Jiabin ; Hu, Yongtao ; Chen, Jing ; Wang, Haojie ; Xu, Wenlong ; Liang, Chaozhao ; Ma, Wenming
57
项与 前列腺炎 相关的新闻(医药)2025-02-19
·药咖荟
肿瘤标志物(tumor marker,TM)很多,但每个指标什么意义,适用于什么肿瘤?笔者对其进行了总结,希望对大家有所帮助。
一、常见、通用的肿瘤血清 TM
1、癌胚抗原(CEA)
CEA升高常见于大肠癌、胰腺癌、胃癌、乳腺癌、甲状腺髓样癌、肝癌、肺癌、卵巢癌、泌尿系肿瘤等。但吸烟、妊娠期和心血管疾病、糖尿病、肠道憩室炎、直肠息肉、结肠炎、胰腺炎、肝硬化、肝炎、肺部疾病等,15%~53%的患者血清CEA也会升高,所以CEA不是恶性肿瘤的特异性标志,在诊断上只有辅助价值。
大量临床实践证实,术前或治疗前CEA浓度能明确预示肿瘤的状态、存活期及有无手术指征等。术前CEA浓度越低,说明病期越早,肿瘤转移、复发的可能越小,其生存时间越长;反之,术前CEA浓度越高说明病期较晚,难于切除,预后差。
CEA检测还可对经手术或其他方法治疗使CEA恢复正常的病人,进行长期随访,监测其复发和转移。通常采用以下方案:术后第六周一次;术后三年内,每月一次;3~5年每三月一次;5~7年每半年一次;7年后一年一次。若发现升高,两周后再测一次,两次都升高则提示复发和转移。
正常参考值≤5ng/mL
2、癌抗原125(CA125)
CA125最常见于上皮性卵巢肿瘤(浆液性肿瘤)患者的血清中,其诊断的敏感性较高,但特异性较差。黏液性卵巢肿瘤中不存在。80%的卵巢上皮性肿瘤患者血清CA125升高,但近半数的早期病例并不升高,故不单独用于卵巢上皮性癌的早期诊断。90%患者血清CA125与病程进展有关,故多用于病情检测和疗效评估。95%的健康成年妇女CA125的水平≤40U/ml,若升高至正常值的2倍以上应引起重视。
各种恶性肿瘤引起的腹水中也可见CA125升高。CA125升高也可见于多种妇科良性疾病,如卵巢囊肿、子宫内膜病、宫颈炎及子宫肌瘤、胃肠道癌、肝硬化、肝炎等。
正常参考值<35u/mL。
3、癌抗原15-3(CA15-3)
癌抗原15-3是乳腺癌的辅助诊断指标,但在乳腺癌早期敏感性不高。早期阳性率为60%,转移性乳腺癌阳性率为80%。癌抗原15-3也是术后随访,监测肿瘤复发、转移的指标。
增高:见于乳腺癌、肺癌、结肠癌、宫颈癌等。乳腺、卵巢等非恶性肿瘤阳性率一般低于10%。
正常参考值:≤25 U/ml
4、癌抗原19-9(CA19-9)
血清癌抗原19-9可作为胰腺癌。胆囊癌等恶性肿瘤的辅助诊断指标。胚胎期胎儿的胰腺、胆囊、肝、肠等组织存在这种抗原,正常人体组织中含量很低;在消化道恶性肿瘤,尤其是胰腺癌、胆囊癌病人血清中,癌抗原19-9含量明显增高,但早期诊断价值不大,主要作为病情监测和预示复发的指标。此外,对消化道疾病鉴别诊断(如胰腺癌与胰腺炎。胃癌与胃溃疡)亦有一定价值。
增高:见于胰腺癌、胆囊癌、胃癌、结肠癌、肝癌等;急性胰腺炎、胆囊炎、肝炎等也有不同程度的升高。
正常参考值:≤27 U/ml
5、癌抗原50(CA50)
癌抗原50是一种非特异性的广谱肿瘤标志物,与癌抗原19-9有一定的交叉抗原性,主要用于胰腺癌、结肠/直肠癌、胃癌的辅助诊断,其中胰腺癌病人增高最明显。
增高:见于胰腺癌(阳性率可达87%)、结肠/直肠癌、胃癌、肺癌。肝癌。卵巢癌、乳腺癌等恶性肿瘤;溃疡性结肠炎、肝硬化、黑色素瘤、淋巴瘤、自身免疫性疾病等也增高。
另有报导萎缩性胃炎患者胃液CA50的浓度与正常人比较有显著改变。通常认为萎缩性胃炎是癌前高危期,因此CA50可作为癌前诊断指标之一。在胰腺炎、结肠炎和肺炎发病时,CA50也会升高,但随炎症消除而下降。
正常参考值:0~20 U/ml
6、癌抗原242(CA242)
CA242是一种新的肿瘤相关抗原,当消化道发生肿瘤时,其含量升高。对胰腺癌、结直肠癌有较高的敏感性与特异性,分别有86 %和62 %的阳性检出率,对肺癌、乳腺癌也有一定的阳性检出率。用于胰腺癌和良性肝胆疾病的鉴别诊断及预后,也用于结直肠癌病人术前预后及复发鉴别。
CEA与CA242联合检测可提高敏感性,与单独采用CEA检测相比,对结肠癌可提高40~70%,对直肠癌提高达到47~62%。CEA与CA242无相关性,具有独立的诊断价值,且二者之间具有互补性。
正常参考值:0~20 U/ml
7、小细胞肺癌相关抗原(神经元特异性烯醇化酶,NSE)
NSE被认为是监测小细胞肺癌的首选标志物,60~80%的小细胞肺癌患者NSE升高。在缓解期,80~96%的患者NSE含量正常,如NSE升高,提示复发。小细胞肺癌患者首轮化疗后24~72小时内,由于肿瘤细胞的分解,NSE呈一过性升高。因此,NSE是监测小细胞肺癌疗效与病程的有效标志物,并能提供有价值的预后信息。
NSE也可作为神经母细胞瘤的标志物,对该病的早期诊断具有较高的临床应用价值。神经母细胞瘤患者的尿中NSE水平也有一定升高,治疗后血清NSE水平降至正常。血清NSE水平的测定对于神经母细胞瘤的监测疗效和预报复发均具有重要参考价值,比测定尿液中儿茶酚胺的代谢物更有意义。
另外对胺前体摄取脱羧细胞瘤、精原细胞瘤及其它脑肿瘤的诊断也有重要意义。
正常参考值:0~16.3 ng/ml
8、鳞状细胞癌抗原(SCC)
鳞状细胞癌抗原(SCC)是一种特异性很好而且是最早用于诊断鳞癌的肿瘤标志物。SCC在正常的鳞状上皮细胞中抑制细胞凋亡和参与鳞状上皮层的分化,在肿瘤细胞中参与肿瘤的生长,它有助于所有鳞状上皮细胞起源癌的诊断和监测,例如:子宫颈癌、肺癌(非小细胞肺癌)、头颈部癌、食管癌、鼻咽癌以及外阴部鳞状细胞癌等。这些肿瘤患者血清中SCC升高,其浓度随病期的加重而增加。临床上用于监测这些肿瘤的疗效、复发、和转移以及评价预后。
对子宫颈癌有较高的诊断价值:对原发性宫颈鳞癌敏感性为44%~69%;复发癌敏感性为67%~100%,特异性90%~96%;其血清学水平与肿瘤发展、侵犯程度及有否转移相关。在宫颈癌根治术后SCC浓度显著下降;可及早提示复发,50%患者的SCC浓度升高先于临床诊断复发2~5个月,它可以作为独立风险因子加以应用。
辅助诊断肺鳞癌:肺鳞癌阳性率为46.5%,其水平与肿瘤的进展程度相关,它配合CA125、CYFRA21-1和CEA联合检测可提高肺癌患者诊断的灵敏性。
食管鳞癌、鼻咽癌的预测:阳性率随病情发展而上升,对于晚期患者,其灵敏性可达73%,联合检测CYFRA21-1和SCC可以提高检测的灵敏性。III期头颈部癌阳性率为40%,IV期时阳性率增至60%。
其它鳞癌的诊断和监测:头颈癌、外阴癌、膀胱癌、肛管癌、皮肤癌等。
正常参考值:< 1.5μg/L
二、肺癌血清 TM
9、非小细胞肺癌相关抗原(CYFRA 21-1)
CYFRA 21-1是非小细胞肺癌最有价值的血清肿瘤标志物,尤其对鳞状细胞癌患者的早期诊断、疗效观察、预后监测有重要意义。CYFRA 21-1也可用于监测横纹肌浸润性膀胱癌的病程,特别是对预计膀胱癌的复发具有较大价值。如果肿瘤治疗效果好,CYFRA 21-1的水平会很快下降或恢复到正常水平,在疾病的发展过程中,CYFRA 21-1值的变化常常早于临床症状和影像检查。
CYFRA 21-1与良性肺部疾病(肺炎、结核、慢性支气管炎、支气管哮喘、肺气肿)的鉴别特异性比较好。
正常参考值:0~3.3 ng/ml
10、胃泌素释放肽前体(ProGRP)
胃泌素前体释放肽是一种新的小细胞肺癌标志物。PROGRP是脑肠激素的一种,是小细胞肺癌增殖因子胃泌素释放肽的前体。
PROGRP作为小细胞肺癌标志物有以下特点:
针对小细胞肺癌的特异性非常高;
较早期的病例有较高的阳性率;
健康者与患者血中浓度差异很大,因而检测的可靠性很高。
具体应用:
肺腺癌:CEA(87%)、CA153
肺鳞癌:SCC、CYFRA21-1
小细胞肺癌:pro-GRP、NSE
非小细胞肺癌三联:CYFRA21-1 + CEA + p53
肺转移:CA199、CA153
复发:CEA
三、胃癌血清 TM
11、癌抗原72-4(CA72-4)
CA72-4是目前诊断胃癌的最佳肿瘤标志物之一,对胃癌具有较高的特异性,其敏感性可达28-80%,若与CA19-9及CEA联合检测可以监测70 %以上的胃癌。CA72-4水平与胃癌的分期有明显的相关性,一般在胃癌的Ⅲ-Ⅳ期增高,对伴有转移的胃癌病人,CA72-4的阳性率更远远高于非转移者。CA72-4水平在术后可迅速下降至正常。在70%的复发病例中,CA72-4浓度首先升高。与其它标志物相比,CA72-4最主要的优势是其对良性病变的鉴别诊断有极高的特异性,在众多的良性胃病患者中,其检出率仅0.7 %。
CA72-4对其他胃肠道癌、乳腺癌、肺癌、卵巢癌也有不同程度的检出率。CA72-4与CA125联合检测,作为诊断原发性及复发性卵巢肿瘤的标志,特异性可达100 %。
正常参考值:≤6.9 U/ml
没有一种能满足早期诊断的敏感度和特异性,通常联合诊断。癌前病变和初期:CA199、CA242、CA724、CEA 升高。
四、结直肠癌血清 TM
12、内皮细胞特异分子-1(ESM-1)
ESM-1(内皮细胞特异分子-1)正常<37.0 ng/mL,结直肠癌敏感度 90.91%、特异度 95%,随TNM分期升高而上升。
CEA、CA242、CA199 三者较敏感,然而联合检测不优于单一 CEA。
五、肝癌血清 TM
13、甲胎蛋白(AFP)
AFP(甲胎蛋白) 正常≤20ng/mL,无肝病活动、排除妊娠和生殖腺胚胎癌,≥400ng/mL 持续 1 月或≥200ng/mL 持续 2 月者,结合影像检查可诊断肝癌。肝硬化、肝炎患者中 AFP 也会升高,但一般不超过 300 ng/ml。
甲胎蛋白主要由胎儿肝细胞及卵黄囊合成。甲胎蛋白在胎儿血液循环中具有较高的浓度,出生后则下降,至生后2~3月甲胎蛋白基本被白蛋白替代,血液中较难检出,故在成人血清中含量极低。甲胎蛋白具有很多重要的生理功能,包括运输功能、作为生长调节因子的双向调节功能、免疫抑制、T淋巴细胞诱导凋亡等。
甲胎蛋白与肝癌及多种肿瘤的发生发展密切相关,在多种肿瘤中均可表现出较高浓度,可作为多种肿瘤的阳性检测指标。目前临床上主要作为原发性肝癌的血清标志物,用于原发性肝癌的诊断及疗效监测。在转移性肝癌中,AFP值一般低于350~400 ng/ml。
14、α-L-岩藻糖苷酶(AFU)
AFU是对原发性肝细胞性肝癌检测的又一敏感、特异的新标志物。原发性肝癌患者血清AFU活力显著高于其它各类疾患(包括良、恶性肿瘤)。血清AFU活性动态曲线对判断肝癌治疗效果、估计预后和预报复发有着极其重要的意义,甚至优于AFP。但是,值得提出的是,血清AFU活力测定在某些转移性肝癌、肺癌、乳腺癌、卵巢或子宫癌之间有一些重叠,甚至在某些非肿瘤性疾患如肝硬化、慢性肝炎和消化道出血等也有轻度升高,在使用AFU时应与AFP同时测定,可提高原发性肝癌的诊断率,有较好的互补作用。
正常参考值:0~40 IU/L
六、前列腺癌血清 TM
15、总前列腺特异性抗原(TPSA)
PSA是前列腺癌的特异性标志物,也是目前公认的唯一具有器官特异性肿瘤标志物。血清TPSA升高一般提示前列腺存在病变(前列腺炎、良性增生或癌症)。血清PSA是检测和早期发现前列腺癌最重要的指标之一,血清TPSA定量的阳性临界值为大于10 μg/L,前列腺癌的诊断特异性达90%~97%。TPSA也可用于高危人群前列腺癌的筛选与早期诊断,是第一个由美国癌症协会推荐用于筛查50岁以上男性前列腺癌的肿瘤标志物。
TPSA测定还可用于监测前列腺癌患者或接受激素治疗患者的病情及疗效,90%前列腺癌术后患者的血清TPSA值可降至不能检出的痕量水平,若术后血清TPSA值升高,提示有残存肿瘤。放疗后疗效显著者,50%以上患者在2个月内血清TPSA降至正常。
正常参考值:≤4.400 ng/ml
16、游离前列腺特异性抗原(FPSA)
单项的血清总PSA(TPSA)测定不能明确鉴别前列腺癌和良性的前列腺增生,主要是因为在浓度2~20 ng/ml范围内,二组病人有交叉。而FPSA/TPSA不受此因素及年龄的影响,通过FPSA/TPSA比值达到鉴别前列腺癌或良性的前列腺增生的目的。前列腺癌患者的FPSA/TPSA比值明显偏低,良性的前列腺增生患者的FPSA/TPSA比值显著增高。FPSA/TPSA界限指定为0.15,低于该值高度怀疑前列腺癌,其诊断敏感性为90.9%,特异性为87.5%,准确性为88.6%,明显优于TPSA单独测定。
FPSA检测主要适用于未经治疗、TPSA值为2~20 ng/ml病人,当TPSA值低于2 ng/ml或高于20 ng/ml时,FPSA/TPSA比值并不能用于鉴别前列腺癌和良性的前列腺增生。
正常参考值:≤1.000ng/ml
FPSA/TPSA:> 0.15
17、前列腺酸性磷酸酶(PAP)
前列腺癌血清PAP升高,是前列腺癌诊断、分期、疗效观察及预后的重要指标。前列腺炎和前列腺增生PAP也有一定程度的增高。
七、卵巢癌血清 TM
18、人附睾蛋白4(HE4)
HE4对于卵巢癌单项特异性、敏感性、准确度较高,CA125 对浆液性卵巢癌的敏感度高于粘液性。
卵巢癌CA125、CA153、AFP、HE4(人附睾蛋白4)较敏感。
八、膀胱癌尿液 TM
19、核基质蛋白-22(NMP-22)
NMP-22(NuclearMatrixProtein-22)是细胞核骨架的组成成分,与细胞的DNA复制、RNA合成、基因表达调控、激素结合等密切相关。膀胱癌中大量肿瘤细胞凋亡将NMP22释放入尿,尿中NMP22可增高达25倍。以10kU/mL为临界值,对膀胱癌诊断的敏感度为70%,特异度78.5%。用于浸润性膀胱癌诊断的敏感度为100%。
九、生殖肿瘤血清 TM
20、人绒毛促性腺激素(HCG)
非妊娠情况下正常<8U/L,肿瘤患者,一般检测β亚单位——β-HCG。HCG是男性睾丸肿瘤和女性恶性滋养细胞肿瘤(葡萄胎、侵袭性葡萄胎、绒毛膜癌)最基本的标记物。
十、其他TM
21、EB病毒抗体(EBV-VCA)
EB病毒阳性、鼻咽癌家族史、鼻咽癌的高发区、身体免疫力低下,都可能是患鼻咽癌的高危因素。从理论上讲,如EB病毒检查阳性者,仅是代表患者以前曾经受过EB病毒感染,但它是否是鼻咽癌发病的直接原因,目前尚无定论。但临床实践,科学研究表明,阳性者患鼻咽癌的机会比阴性者大得多。
EBV-VCA抗体临床意义:VCA-IgA≥1:10为阳性,说明感染过EB病毒(多在半年前或很久很久前),临床上与鼻咽癌、胸腺淋巴上皮癌、胃癌、直肠癌、类风湿性关节炎、非甲非乙型肝炎、红斑狼疮、干燥综合症、Burkitt氏淋巴瘤、免疫缺陷宿主的淋巴瘤等疾病有关。VCA-IgM≥1:5为阳性,说明有近期感染,(感染后多在2~3周该抗体升高,在体内持续时间不等)临床上与不明原因的发烧、乏力、传染性单核细胞增多症、紫癜、抽风、川畸病、口腔脱皮等自身免疫病有关;VCA-IgG≥1:80以上者,说明EBV被激活或激活了其它病毒基因及某些细胞基因,可作为EBV或其它病毒感染的参考招标。
正常参考值:EBV-VCA抗体阴性
22、肿瘤相关物质(TSGF)
TSGF肿瘤相关物质联合检测(原名恶性肿瘤特异性生长因子)是一种可以简便快速地用于恶性肿瘤早期辅助诊断的新型的肿瘤标志物,对疗效观察、人群查体亦有很高的应用价值。由糖类物质构成的糖脂、糖蛋白、寡聚糖等广泛分布于细胞内外和各种体液中,在细胞发生癌变时其代谢紊乱可引起体液中的含量升高,是国际公认的肿瘤标志物;氨基酸及其代谢产物也由于其瘤种特异性小而适用于普查筛选。几种小分子的肿瘤标志物组合在一起合称为TSGF,由于TSGF含量在肿瘤早期血清中即会明显升高,这一特性使其成为广谱恶性肿瘤早期辅助诊断的理想指标。
TSGF也是癌症病人治疗效果及动态随访指标,临床应用资料表明癌症病人治疗前TSGF检测值显著升高,经有效治疗后,患者血清中TSGF值明显下降,甚至降至正常水平;治疗无效或病情恶化、复发或转移的患者,TSGF值反而上升。因此TSGF在疗效观察方面有重要价值,治疗过程中可根据TSGF的检测结果及时调整治疗方案,以期达到最佳治疗效果。
部分急性炎症(肝炎、肺炎等)、自身免疫性疾病如系统性红斑狼疮、类风湿等病症可产生交叉反应,引起假阳性。晚期癌症患者TSGF含量可能低于临界值。
正常参考值:<64U/ml为阴性
23、铁蛋白(SF)
铁蛋白升高可见于下列肿瘤:急性白血病、何杰金氏病、肺癌、结肠癌、肝癌和前列腺癌。检测铁蛋白对肝脏转移性肿瘤有诊断价值,76%的肝转移病人铁蛋白含量高于400μg/L,当肝癌时,AFP测定值较低的情况下,可用铁蛋白测定值补充,以提高诊断率。在色素沉着、炎症、肝炎时铁蛋白也会升高。升高的原因可能是由于细胞坏死,红细胞生成被阻断或肿瘤组织中合成增多。
正常参考值:男性:22~322 μg/L 女性:13~150 μg/L
24、β2-微球蛋(β2-MG)
β2-MG是恶性肿瘤的辅助标志物,也是一些肿瘤细胞上的肿瘤相关抗原。在恶性血液病或其它实质性癌瘤中,突变细胞合成和分泌β2-MG,可使病人血清中浓度显著上升,在淋巴系统肿瘤如慢性淋巴细胞白血病、淋巴细胞肉瘤、多发性骨髓瘤等中尤为明显,在肺癌、乳腺癌、胃肠道癌及子宫颈癌等中也可见增高。由于在肿瘤早期,血清β2-MG可明显高于正常值,故有助于鉴别良、恶性肿瘤。有报道发现恶性疾病时β2-MG在腹水中与血清中的比例明显相关,若两者比值大于1.3时,即考虑为癌肿的表现。
血清β2-MG不但可以在肾功能衰竭、多种血液系统疾病及炎症时升高,而且在多种疾病中均可增高,故应排除由于某些炎症性疾病或肾小球滤过功能减低所致的血清β2-MG增高。脑脊液中β2-MG的检测对脑膜白血病的诊断有特别的意义。
正常参考值:1.58~3.55 μg/ml
25、胰胚胎抗原(POA)
胰胚胎抗原是胰腺癌的又一新型、敏感、特异的新标志物,胰腺癌的POA的阳性率为95%,其血清含量大于20U/ml,当肝癌、大肠癌、胃癌等恶性肿瘤时也会使POA升高,但阳性率较低。
正常参考值:0~7 U/ml
声明
本微信公众号对所有原创、转载的内容、陈述、观点判断均保持中立,推送内容仅供公益性分享,部分转载作品、图片如有作者来源标记有误或涉及侵权,请联系小编删除。
更多优质内容,欢迎关注↓
媒体合作
投稿转载/资料领取/加入社群 请添加药小咖
与/智/者/同/行 为/创/新/赋/能
临床研究
2025-02-18
·摩熵医药
注:本文不构成任何投资意见和建议,以官方/公司公告为准;本文仅作医疗健康相关药物介绍,非治疗方案推荐(若涉及),不代表平台立场。任何文章转载需要得到授权。
近日,据国家药监局官网显示,陕西摩美得气血和制药的双石通淋胶囊和山东华信制药集团的龙香平喘胶囊,这两款备受瞩目的中药品种保护申请同日获受理。据摩熵医药数据库显示,双石通淋胶囊在2024年(Q1~Q3)全国院内市场的销售额超1亿元。
截图来源:NMPA官网
双石通淋胶囊是陕西摩美得气血和制药的独家中成药品品种,主要是清热利尿,化浊通淋。用于慢性前列腺炎湿热壅阻证。症见尿道灼热、小便频急、尿后余沥不尽、尿后滴白、阴部潮湿、会阴、少腹、腰骶部疼痛或不适,舌质红苔黄,脉弦或弦滑等。据摩熵医药数据库显示,双石通淋胶囊在2024年(Q1~Q3)全国院内市场的销售额超1亿元,同比增长达6.95%。
截图来源:摩熵医药全国医院销售数据库
陕西摩美得气血和制药在中药领域的深耕细作。截至目前,陕西摩美得气血和制药(含陕西长鸣制药)有104款中药品种获批上市,其中西帕依固龈泡腾片、绞股蓝总苷滴丸、复方地锦胶囊、西帕依麦孜彼子颗粒等26款为独家中药品种。
截图来源:摩熵医药中国药品批文数据库
今年以来,国家药监局官网在中药保护领域动作频频,已发布两批中药保护品种受理公示,共计受理了5款独家中药品种的保护申请。除了上述的双石通淋胶囊和龙香平喘胶囊外,还包括清华德人西安幸福制药的五灵胶囊、四川禾亿制药的板蓝根滴眼液以及武汉科兴医药的骨参片,其中清华德人西安幸福制药的五灵胶囊在2024年(Q1~Q3)全国院内市场的销售额超2亿元。
截图来源:NMPA官网
小结
陕西摩美得气血和制药及山东华信制药集团等企业在中药领域的深耕细作,本次双石通淋胶囊和龙香平喘胶囊等独家中药品种获得保护申请受理,不仅丰富了中药产品线,也为患者提供了更多有效的治疗选择。随着国家对中药产业的支持力度不断加大,相信中药产业将迎来更加美好的发展前景。
END
本文为原创文章,转载请留言获取授权
近期热门资源获取
CGT产业现状与未来趋势蓝皮书-202406
中药行业现状与未来趋势白皮书-202407
2023年医药企业综合实力排行榜-202408
跨越国界,引领创新:中国药企出海的布局实践-202408
专利即将到期五大重磅小分子药品,国内仿制药“战况”几何?-202409
中国放射性药物市场现状分析报告-202410
合成生物产业发展前景及中国合成生物产业链上中下游企业分析-202410
中国合成生物学创投市场分析报告-202410
中国糖尿病临床诊疗与药物多渠道市场数据分析-202411
基于剂型改良的复杂注射剂分析--微球篇-202411
2024医美注射材料市场发展分析报告-20241213
国家药品集采跟踪报告-前9批次集采回顾与展望-202411
近期更多摩熵咨询热门报告,识别下方二维码领取
联系我们,体验摩熵医药更多专业服务
会议
合作
园区
服务
数据库
咨询
定制
服务
媒体
合作
👆👆👆点击上方图片,即可开启摩熵化学数据查询
点击阅读原文,申请摩熵医药企业版免费试用!
专利到期
2025-02-17
·米内网
精彩内容
近日,国家药监局官网显示,双石通淋胶囊申请中药品种保护获受理。该产品是陕西摩美得气血和制药的独家品种,米内网数据显示,双石通淋胶囊在2023年中国三大终端六大市场(统计范围见文末)销售额超过1亿元。
来源:米内网一键检索
双石通淋胶囊具有清热利湿,化浊通淋的作用,临床上主要用于慢性前列腺炎属湿热壅阻症的治疗。
近年中国零售药店终端双石通淋胶囊销售情况(单位:万元)
来源:米内网格局数据库
米内网数据显示,双石通淋胶囊在2023年中国三大终端六大市场销售额超过1亿元。其中,院内市场(公立医院终端+公立基层医疗终端)是主力销售渠道,院外市场(零售药店:城市实体药店+网上药店)则快速增长,均保持双位数增速。
资料显示,陕西摩美得气血和制药是一家集产、学、研、销为一体的中药高新技术企业,拥有108个批准文号,18个独家产品,10个独家剂型产品,产品涵盖心脑血管、消化、妇儿、泌尿等领域。
今年以来受理的中药品种保护申请
今年以来,国家药监局官网共发布2个中药保护品种受理公示,陕西摩美得气血和制药的双石通淋胶囊、山东华信制药集团的龙香平喘胶囊、清华德人西安幸福制药的五灵胶囊等5个均为独家品种。
资料来源:米内网数据库、国家药监局官网等
注:米内网《中国三大终端六大市场药品竞争格局》,统计范围是:城市公立医院和县级公立医院、城市社区中心和乡镇卫生院、城市实体药店和网上药店,不含民营医院、私人诊所、村卫生室,不含县乡村药店;上述销售额以产品在终端的平均零售价计算。如有疏漏,欢迎指正!
本文为原创稿件,转载请注明来源和作者,否则将追究侵权责任。投稿及报料请发邮件到872470254@qq.com稿件要求详询米内微信首页菜单栏商务及内容合作可联系QQ:412539092
【分享、点赞、在看】点一点不失联哦
分析
对领域进行一次全面的分析。
登录
或
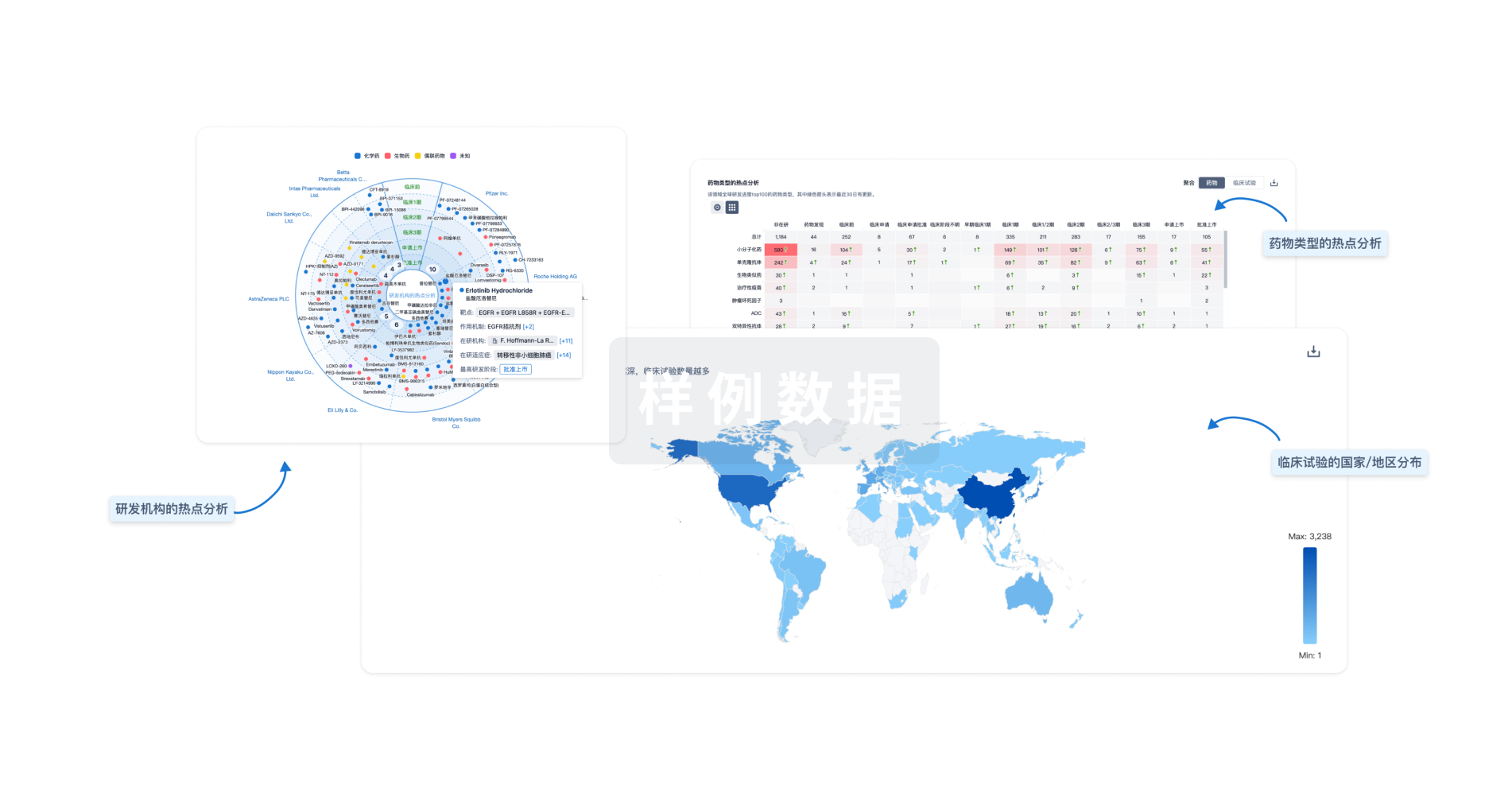
Eureka LS:
全新生物医药AI Agent 覆盖科研全链路,让突破性发现快人一步
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用

