更新于:2024-11-01
Gray Platelet Syndrome
灰色血小板综合征
更新于:2024-11-01
基本信息
别名 Alpha storage pool deficiency、BDPLT4、BLEEDING DISORDER, PLATELET-TYPE, 4 + [26] |
简介 A rare, inherited platelet disorder characterized by a selective deficiency in the number and contents of platelet alpha-granules. It is associated with THROMBOCYTOPENIA, enlarged platelets, and prolonged bleeding time. |
关联
5
项与 灰色血小板综合征 相关的临床试验Evaluation of the German directive for specialized outpatient palliative care (SAPV): Outcomes, interactions, regional differences - SAVOIR / subproject 4
开始日期2018-05-16 |
申办/合作机构- |
Quantitative Targeted Proteomics Detected by Mass Spectrometry With Whole Genome (DNA) and Whole Transcriptome (RNA) Sequencing in Advanced Cancers
Among patients with advanced (metastatic) cancers, detailed characterizations of the tumor utilizing genomic and proteonomic techniques may help guide treatment. It, however, remains unclear if these new diagnostic technologies truly influence clinical and economic outcomes. This study will evaluate if patients treated according to the results of the NantHealth GPS Cancer test achieve optimal outcomes compared to patients whose treatment are discordant with GPS Cancer recommendations.
开始日期2017-02-28 |
申办/合作机构  Cota, Inc. Cota, Inc. [+2] |
Patient-oriented, evidence-based, search-strategy learn, develop and to use
开始日期2006-01-01 |
申办/合作机构- |
100 项与 灰色血小板综合征 相关的临床结果
登录后查看更多信息
100 项与 灰色血小板综合征 相关的转化医学
登录后查看更多信息
0 项与 灰色血小板综合征 相关的专利(医药)
登录后查看更多信息
545
项与 灰色血小板综合征 相关的文献(医药)2024-09-06·Cureus
Laparoscopic Cholecystectomy for Gallbladder Polyps: Is It Overtreatment?
Article
作者: Ozel, Yahya ; Kara, Yalçın Burak
2024-09-01·Cureus
Genitopatellar Syndrome With a Novel Variant in the KAT6B Gene: Supporting Spectrum Delineation.
Article
作者: Mahfooz, Naeem ; Mierzwa, Adam ; Back, Warren
2024-07-21·Blood transfusion = Trasfusione del sangue
Evaluation of an automated platelet aggregation method for detection of congenital or acquired platelet function defects.
Article
作者: Artoni, Andrea ; Padovan, Lidia ; La Marca, Silvia ; Shinohara, Sho ; Lecchi, Anna ; Arai, Nobuo ; Capecchi, Marco ; Peyvandi, Flora
2
项与 灰色血小板综合征 相关的新闻(医药)2024-01-09
·药明康德
▎药明康德内容团队编辑本期看点1. 用于治疗与广泛发育性和癫痫性脑病(DEE)相关癫痫发作的新型5-HT2C受体超激动剂bexicaserin(LP352)的1b/2a期临床试验达主要终点,使可数运动性癫痫发作频率较基线中位减少了53.3%。2. 肿瘤免疫治疗新药Galinpepimut-S(GPS)联用PD-1抑制剂纳武利尤单抗治疗恶性胸膜间皮瘤,患者的中位总生存期(OS)约为接受标准治疗的患者的2.5倍。3. Orion公司与默沙东(MSD)合作开发的CYP11A1抑制剂早期临床结果积极,超过半数携带雄激素受体(AR)配体结合域(LBD)突变的转移性去势抵抗性前列腺癌(mCRPC)患者的前列腺特异性抗原(PSA)水平下降≥50%。4. 直接将基因疗法送入大脑的帕金森病创新疗法达到临床试验主要终点。药明康德内容团队整理Bexicaserin(LP352):公布1b/2a期临床试验数据 Longboard Pharmaceuticals公司今天公布了PACIFIC研究的积极数据,该研究评估其在研疗法bexicaserin(LP352)用于治疗与广泛发育性和癫痫性脑病相关癫痫发作的疗效与安全性。Bexicaserin是一款高选择性、口服、新型5-HT2C受体超激动剂,为潜在的“best-in-class”疗法。结果显示,接受bexicaserin治疗的可评估受试者(n=35)的可数运动性癫痫发作频率(主要疗效终点)较基线的中位变化为降低53.3%,而接受安慰剂治疗的受试者(n=9)则降低20.8%,这代表经安慰剂校正的癫痫发作频率降低32.5%。Dravet综合征(DS)、Lennox-Gastaut综合征(LGS)和其他DEE亚组队列可数运动性癫痫发作频率较基线的中位变化分别降低72.1%、48.1%和61.2%。Bexicaserin表现出良好的安全性和耐受性结果。观察到的最常见不良事件(AE)为嗜睡、食欲减退、便秘、腹泻。Longboard计划将迅速推进bexicaserin的全球3期试验。Galinpepimut-S:公布1期临床试验的新数据 SELLAS Life Sciences Group公布了其靶向WT1蛋白的潜在“first-in-class”肿瘤免疫治疗新药Galinpepimut-S联用PD-1抑制剂纳武利尤单抗用于治疗恶性胸膜间皮瘤患者的1期临床新数据,这些患者的肿瘤普遍表达WT1。该药由4条多肽链构成,抗原表位多达25个,适用于全球范围内绝大多数人类白细胞组织相容性抗原(HLA)类型,能够激发自身免疫系统对WT1抗原强烈的免疫反应。研究结果显示,该试验达到了安全性和疗效的主要终点,并观察到了联合疗法的临床活性和患者生存率的提高。接受联合疗法的患者的中位OS为70.3周,而接受标准治疗的复发/难治性患者的中位OS约为28周。对GPS没有免疫反应的患者的中位OS为9.0个月,对GPS有免疫反应的患者的中位OS为27.8个月。全部患者的中位无进展生存期(PFS)为11.9周,疾病控制率(DCR)为30%。ODM-208(MK-5684):公布1/2期临床试验数据 ODM-208是Orion公司与默沙东合作开发的一种用于治疗前列腺癌等激素依赖性癌症的口服非甾体类选择性抑制剂。通过抑制CYP11A1酶的活性,ODM-208可以抑制所有类固醇激素和可能激活雄激素受体信号通路的激素前体的产生。此次公布结果的1/2期试验评估了ODM-208对先前接受过多线治疗的mCRPC患者的安全性和有效性。结果显示,在1期试验中,14/19(73.7%)名AR LBD突变患者和2/23(8.7%)名AR野生型患者的PSA水平下降了50%或以上。2a期试验中,24/45(53.3%)名AR LBD突变患者的PSA水平下降了50%或以上。46/53(87%)名患者的中位循环睾酮水平在接受ODM-208治疗的第一周内从基线时的3.0 ng/dl降至检测不到的水平。大多数患者耐受性良好,与治疗相关的肾上腺功能不全是最常见的安全性问题。总体而言,1期试验有17名(36.2%)患者和2a期试验有6名(13.3%)患者出现肾上腺功能不全,需要调整激素替代疗法和/或额外补充治疗,之后通常会继续接受ODM-208治疗。AB-1005(AAV2-GDNF):公布1b期临床试验数据 拜耳(Bayer)公司旗下Asklepios BioPharmaceutical(AskBio)公司宣布,治疗帕金森病的在研基因疗法AB-1005(AAV2-GDNF)在1b期临床试验中达到主要终点,在11名接受治疗的患者中表现出良好的安全性。基于这一结果,两家公司计划在2024年上半年启动2期临床试验。AB-1005是一种基于腺相关病毒血清型2(AAV2)载体的在研基因疗法,含有编码人胶质细胞源性神经营养因子(GDNF)的转基因。通过磁共振成像(MRI)监测的对流增强给药方式直接进行神经外科注射后,GDNF可以在大脑局部区域实现稳定和连续的表达。GNSC-001:启动1b期临床试验Genascence Corporation公司宣布启动1b期临床试验,评估其在研基因疗法GNSC-001治疗膝盖骨关节炎的安全性和效果,试验预计招募约50名患者。GNSC-001是一款使用重组腺相关病毒载体表达编码IL-1Ra蛋白转基因的在研基因疗法。IL-1Ra蛋白是一种阻断IL-1信号传导的天然蛋白。IL-1被认为是介导骨关节炎病例发生的关键因子,可导致炎症、关节痛和软骨损害。GNSC-001旨在通过一次注射到关节中,提供长期持久的IL-1信号通路抑制。SC262:IND申请获得FDA许可Sana Biotechnology公司宣布,在研细胞疗法SC262的IND申请获得美国FDA的许可,用于在临床试验中治疗复发或难治性B细胞恶性肿瘤。SC262是基于Sana公司的低免疫(hypoimmune)平台设计的靶向CD22的CAR-T细胞疗法。Sana公司的低免疫平台旨在克服同种异体细胞的免疫排斥,让同种异体CAR-T细胞疗法具有更长的持久性,带来更长的完全缓解。这一平台通过基因编辑扰乱1型和2型主要MHC的表达,让移植细胞不被适应性免疫系统发现。不过这同时通常会导致它们更容易被先天免疫系统发现,尤其是自然杀伤(NK)细胞。Sana的平台因此提供了逃避先天免疫细胞杀伤的方法,包括过度表达CD47,这种蛋白防止细胞被NK细胞或者巨噬细胞杀伤。SENTI-202:IND申请获得FDA许可Senti Biosciences公司宣布其现货型嵌合抗原受体(CAR)NK细胞疗法候选疗法SENTI-202的IND申请获得了美国FDA的许可。Senti Bio公司开发的细胞疗法通过给细胞加上逻辑回路,让细胞更为“智能”地区分癌细胞和健康细胞。SENTI-202是一款新一代CAR-NK细胞疗法。它携带了两种不同的逻辑回路,“OR”回路让它能够识别癌细胞表面表达的CD33或FLT3抗原,从而具有更高的抗癌潜力。而“NOT”回路在识别造血干细胞表面表达的EMCN抗原时会抑制疗法的活性,从而避免SENTI-202杀伤健康造血干细胞。这款CAR-NK疗法还表达可控释放的IL-15蛋白,进一步增强NK细胞的活性。在临床前研究中,它已经表现出良好的抗癌活性和显著降低的肿瘤外毒性。Senti公司计划在2024年第二季度为1期临床试验的首例患者进行给药,预计在2024年年底获得初步的临床疗效数据,2025年获得耐久性数据。DA-1726:向FDA提交IND申请DA-1726是NeuroBo Pharmaceuticals公司开发的一种新型胃泌酸调节素(oxyntomodulin,OXM)类似物,具有胰高血糖素样肽1受体(GLP-1R)和胰高血糖素受体(GCGR)双激动剂的功能,可通过降低食欲和增加能量消耗来减轻体重。DA-1726被设计为每周皮下注射一次,拟开发用于治疗肥胖和非酒精性脂肪性肝炎(NASH)。DA-1726的作用机制明确,在临床前小鼠模型中,其减肥效果优于司美格鲁肽和cotadutide(另一种OXM类似物)。近期,NeuroBo Pharmaceuticals公司已向美国FDA提交了DA-1726的IND申请,以开展1期临床试验。INF904:公布1期临床试验数据InflaRx公司公布了其口服小分子C5aR抑制剂INF904的随机、双盲、安慰剂对照1期试验的初步结果。INF904可通过受体C5aR抑制C5a诱导的信号传导,已在多个临床前疾病模型中显示出抗炎治疗效果。此外,与已上市的C5aR抑制剂不同,体外实验证明INF904对细胞色素P450 3A4/5(CYP3A4/5)酶的抑制作用极小,而CYP3A4/5酶在包括糖皮质激素在内的多种代谢物和药物的代谢过程中发挥着重要作用。此次公布的数据显示,药代动力学(PK)和药效学(PD)数据为INF904的“best-in-class”潜力提供了支持。在14天的给药期间,患者的C5a诱导的中性粒细胞活化阻断率≥90%。此外,在不同剂量下,INF904在体内的暴露水平与剂量之间存在良好的比例关系,并能产生所需的C5a诱导的中性粒细胞活化阻断活性(阻断率>90%)。安全性方面,INF90的耐受性良好,在整个测试剂量范围内重复给药后,受试者没有出现任何值得关注的不良安全事件。PAS-004:IND申请获得FDA许可Pasithea Therapeutics公司宣布,美国FDA批准其大环MEK抑制剂PAS-004的IND申请,以评估PAS-004在MAPK通路驱动的晚期实体肿瘤患者中的应用,这些患者携带RAS、RAF或NF1突变,或有BRAF/MEK抑制剂治疗失败的经历。Pasithea公司预计将于2024年第一季度为首例患者用药。与目前FDA批准的MEK抑制剂不同,PAS-004是大环化合物,环化可增强药物与靶受体的结合,被认为有望改善药代动力学和安全性。PAS-004已在各种疾病的小鼠模型中进行了测试,并完成了临床前测试和动物毒理学研究。此外,FDA还授予PAS-004孤儿药资格,用于治疗1型神经纤维瘤病。新闻稿指出,PAS-004是首个进入人体临床试验的大环MEK抑制剂。大家都在看药明康德为全球生物医药行业提供一体化、端到端的新药研发和生产服务,服务范围涵盖化学药研发和生产、生物学研究、临床前测试和临床试验研发、细胞及基因疗法研发、测试和生产等领域。如您有相关业务需求,欢迎点击下方图片填写具体信息。▲如您有任何业务需求,请长按扫描上方二维码,或点击文末“阅读原文/Read more”,即可访问业务对接平台,填写业务需求信息▲欲了解更多前沿技术在生物医药产业中的应用,请长按扫描上方二维码,即可访问“药明直播间”,观看相关话题的直播讨论与精彩回放参考资料(可上下滑动查看)[1] Senti Bio Announces FDA Clearance of IND Application for SENTI-202 for the Treatment of Relapsed or Refractory Hematologic Malignancies Including Acute Myeloid Leukemia. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2023/12/22/2800521/0/en/Senti-Bio-Announces-FDA-Clearance-of-IND-Application-for-SENTI-202-for-the-Treatment-of-Relapsed-or-Refractory-Hematologic-Malignancies-Including-Acute-Myeloid-Leukemia.html[2] SELLAS Life Sciences Reports Positive Follow-Up Immune Response and Survival Data in Completed Phase 1 Study of Galinpepimut-S Combined with Opdivo® in Advanced Malignant Pleural Mesothelioma. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2023/12/27/2801365/0/en/SELLAS-Life-Sciences-Reports-Positive-Follow-Up-Immune-Response-and-Survival-Data-in-Completed-Phase-1-Study-of-Galinpepimut-S-Combined-with-Opdivo-in-Advanced-Malignant-Pleural-Me.html[3] Kiromic BioPharma Reports Favorable Early Safety and Tolerability Data from First Patient Enrolled in the Phase 1 Deltacel-01 Clinical Trial. Retrieved January 5, 2023, from https://www.businesswire.com/news/home/20240105590033/en/[4] NEJM Evidence publishes results from Phase I/IIa CYPIDES trial with ODM-208. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2023/12/27/2801265/0/en/NEJM-Evidence-publishes-results-from-Phase-I-IIa-CYPIDES-trial-with-ODM-208.html[5] OliX Pharmaceuticals Receives HREC Approval to Initiate Phase 1 Clinical Trial of NASH and Obesity Drug. Retrieved January 5, 2023, from https://www.businesswire.com/news/home/20231222734122/en[6] Oncternal Therapeutics Updates the Status of its Phase 1/2 Study of ONCT-808, a ROR1-Targeting Autologous CAR T, in Patients with Relapsed or Refractory Aggressive B-cell Lymphoma. Retrieved January 5, 2023, from https://investor.oncternal.com/news-releases/news-release-details/oncternal-therapeutics-updates-status-its-phase-12-study-onct[7] Tyra Biosciences Doses First Patient with TYRA-200 and Provides Positive Updates on TYRA-300. Retrieved January 5, 2023, from https://www.prnewswire.com/news-releases/tyra-biosciences-doses-first-patient-with-tyra-200-and-provides-positive-updates-on-tyra-300-302021502.html[8] NeuroBo Pharmaceuticals Submits IND Application to the FDA for a Phase 1 Clinical Trial of DA-1726 for the Treatment of Obesity. Retrieved January 5, 2023, from https://www.prnewswire.com/news-releases/neurobo-pharmaceuticals-submits-ind-application-to-the-fda-for-a-phase-1-clinical-trial-of-da-1726-for-the-treatment-of-obesity-302023115.html[9] NKGen Biotech, Inc. Announces Dosing of First Patient in its Phase 1/2a Trial with Autologous NK Cell Product, SNK01, for the Treatment of Moderate Alzheimer’s Disease. Retrieved January 5, 2023, from https://nkgenbiotech.com/nkgen-biotech-inc-announces-dosing-of-first-patient-in-its-phase-1-2a-trial-with-autologous-nk-cell-product-snk01-for-the-treatment-of-moderate-alzheimers-disease/[10] atai Life Sciences Announces Positive Topline Results from Single Ascending Dose Phase 1 Study with EMP-01 (R-MDMA). Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/02/2802639/0/en/atai-Life-Sciences-Announces-Positive-Topline-Results-from-Single-Ascending-Dose-Phase-1-Study-with-EMP-01-R-MDMA.html[11] Longboard Pharmaceuticals Announces Positive Topline Data from the PACIFIC Study, a Phase 1b/2a Clinical Trial, for Bexicaserin (LP352) in Participants with Developmental and Epileptic Encephalopathies (DEEs). Retrieved January 5, 2023, from https://www.businesswire.com/news/home/20240102733902/en[12] Ubix Therapeutics Receives US FDA Clearance to Proceed with Phase 1 Study of UBX-303-1, an Orally Dosed Small Molecule BTK Degrader for Treatment of Relapsed/refractory B-cell Malignancies. Retrieved January 5, 2023, from http://ubixtrx.com/news/press/59[13] Pasithea Therapeutics Announces FDA Acceptance of IND Application to Evaluate PAS-004 in Advanced Cancer Patients. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/02/2802677/0/en/Pasithea-Therapeutics-Announces-FDA-Acceptance-of-IND-Application-to-Evaluate-PAS-004-in-Advanced-Cancer-Patients.html[14] INmune Bio Announces First Patient Dosed in a Phase 1/2 Study of INKmune™ in Patients with Metastatic Castration-Resistant Prostate Cancer. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/02/2802613/0/en/INmune-Bio-Announces-First-Patient-Dosed-in-a-Phase-1-2-Study-of-INKmune-in-Patients-with-Metastatic-Castration-Resistant-Prostate-Cancer.html[15] BridgeBio announces FDA clearance of IND application for BBO-8520, a first-in-class direct inhibitor of KRASG12C (ON). Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/03/2803197/0/en/BridgeBio-announces-FDA-clearance-of-IND-application-for-BBO-8520-a-first-in-class-direct-inhibitor-of-KRASG12C-ON.html[16] GPN Vaccines Reports Positive Safety and Immunogenicity Data from its Phase 1 Trial of Gamma-PN. Retrieved January 5, 2023, from https://www.prnewswire.com/news-releases/gpn-vaccines-reports-positive-safety-and-immunogenicity-data-from-its-phase-1-trial-of-gamma-pn-302025669.html[17] Genascence Announces Initiation of Phase 1b Clinical Trial of GNSC-001 Gene Therapy for Knee Osteoarthritis (OA). Retrieved January 5, 2023, from https://www.prnewswire.com/news-releases/genascence-announces-initiation-of-phase-1b-clinical-trial-of-gnsc-001-gene-therapy-for-knee-osteoarthritis-oa-302022071.html[18] Ventus Therapeutics Initiates Clinical Testing of VENT-03, a First-in-Class, Orally Administered cGAS Inhibitor. Retrieved January 5, 2023, from https://www.businesswire.com/news/home/20240103586030/en/[19] MAPLIGHT THERAPEUTICS ANNOUNCES COMPLETION OF PHASE 1 CLINICAL TRIAL FOR NOVEL M1/M4 MUSCARINIC AGONIST IN DEVELOPMENT FOR SCHIZOPHRENIA AND ALZHEIMER'S DISEASE PSYCHOSIS. Retrieved January 5, 2023, from https://www.prnewswire.com/news-releases/maplight-therapeutics-announces-completion-of-phase-1-clinical-trial-for-novel-m1m4-muscarinic-agonist-in-development-for-schizophrenia-and-alzheimers-disease-psychosis-302025329.html[20] Sitryx announces collaboration partner Lilly has commenced a Phase 1 study of SIT-011 for chronic autoimmune and inflammatory diseases. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/04/2803717/0/en/Sitryx-announces-collaboration-partner-Lilly-has-commenced-a-Phase-1-study-of-SIT-011-for-chronic-autoimmune-and-inflammatory-diseases.html[21] GlycoMimetics Announces Positive Initial Safety and Pharmacokinetic Results from Phase 1a Healthy Volunteer Study of GMI-1687. Retrieved January 5, 2023, from https://www.businesswire.com/news/home/20240104347772/en[22] InflaRx Announces Positive Topline Results from the Multiple Ascending Dose (MAD) Phase I Study with C5aR Inhibitor INF904. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/04/2803830/0/en/InflaRx-Announces-Positive-Topline-Results-from-the-Multiple-Ascending-Dose-MAD-Phase-I-Study-with-C5aR-Inhibitor-INF904.html[23] Quanta Announces IND Clearance by U.S. FDA for QTX3034, G12D-Preferring Multi-KRAS Inhibitor, and Other Pipeline Updates. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/04/2803965/0/en/Quanta-Announces-IND-Clearance-by-U-S-FDA-for-QTX3034-G12D-Preferring-Multi-KRAS-Inhibitor-and-Other-Pipeline-Updates.html[24] OnQuality Announces FDA Clearance of IND Application for OQL025 for the treatment of EGFR Inhibitor-Induced Acneiform Rash. Retrieved January 5, 2023, from https://www.prnewswire.com/news-releases/onquality-announces-fda-clearance-of-ind-application-for-oql025-for-the-treatment-of-egfr-inhibitor-induced-acneiform-rash-302025647.html[25] Medivir´s licensee, Tango Therapeutics, has dosed the first patient with TNG348, a novel USP1 inhibitor, in a phase 1/2 clinical study. Retrieved January 5, 2023, from https://www.prnewswire.com/news-releases/medivirs-licensee-tango-therapeutics-has-dosed-the-first-patient-with-tng348-a-novel-usp1-inhibitor-in-a-phase-12-clinical-study-302026095.html[26] Kynexis Announces Initiation of First-in-Human Phase 1 Study of KYN-5356, a Potential Treatment for Cognitive Impairment Associated with Schizophrenia. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/04/2803801/0/en/Kynexis-Announces-Initiation-of-First-in-Human-Phase-1-Study-of-KYN-5356-a-Potential-Treatment-for-Cognitive-Impairment-Associated-with-Schizophrenia.html[27] OnKure Announces IND Clearance by U.S. FDA Enabling Phase 1 Initiation for its Mutant Selective PI3Kα inhibitor, OKI-219. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/04/2804053/0/en/OnKure-Announces-IND-Clearance-by-U-S-FDA-Enabling-Phase-1-Initiation-for-its-Mutant-Selective-PI3K%CE%B1-inhibitor-OKI-219.html[28] Sana Biotechnology Announces FDA Clearance of Investigational New Drug Application for SC262, a Hypoimmune-modified, CD22-directed Allogeneic CAR T Therapy, for Patients with Relapsed or Refractory B-cell Malignancies. Retrieved January 5, 2023, from https://www.globenewswire.com/news-release/2024/01/05/2804686/0/en/Sana-Biotechnology-Announces-FDA-Clearance-of-Investigational-New-Drug-Application-for-SC262-a-Hypoimmune-modified-CD22-directed-Allogeneic-CAR-T-Therapy-for-Patients-with-Relapsed.html[29] AskBio Phase Ib trial of AB-1005 gene therapy in patients with Parkinson’s disease meets primary endpoint. Retrieved January 5, 2024, from https://www.bayer.com/media/en-us/askbio-phase-ib-trial-of-ab-1005-gene-therapy-in-patients-with-parkinsons-disease-meets-primary-endpoint/免责声明:药明康德内容团队专注介绍全球生物医药健康研究进展。本文仅作信息交流之目的,文中观点不代表药明康德立场,亦不代表药明康德支持或反对文中观点。本文也不是治疗方案推荐。如需获得治疗方案指导,请前往正规医院就诊。版权说明:本文来自药明康德内容团队,欢迎个人转发至朋友圈,谢绝媒体或机构未经授权以任何形式转载至其他平台。转载授权请在「药明康德」微信公众号回复“转载”,获取转载须知。分享,点赞,在看,聚焦全球生物医药健康创新
临床结果临床3期基因疗法免疫疗法临床2期
2022-11-16
·药时代
点击图片,预约今晚7点半直播!正文共: 2657字 1图预计阅读时间: 7分钟Sellas Life Sciences,一家跟中国药企关系密切的美国biotech公司。其管线里拥有两款产品:一款是从劲方医药引进的CDK9小分子抑制剂;另一款First-in-Class的产品——靶向WT1蛋白的多肽癌症疫苗Galinpepimut-S(GPS)则在两年前被授权给了思路迪医药,由思路迪在大中华区进行开发,中方代号为3D189。2022年11月14日,Sellas公布了GPS针对急性髓性白血病(AML)患者三期临床试验(REGAL研究)的重要更新。消息公布之后,股价当日大跌45%,次日继续下跌12%,两天的累计跌幅超过50%,Sellas市值则直接跌破5000万美元。来源:雪球然而有趣的是,此次公布的消息并非试验失败或试验数据不理想,反而是产品效果太好,受试者的中位OS数据大幅延长,达到了预估的两倍左右,导致试验的持续时间延长,试验方案被迫修改。1曾经的免疫疗法第一靶点GPS是一款创新性肿瘤免疫治疗药物,作用机制上属于癌症疫苗。其靶向的WT1蛋白(Wilms肿瘤蛋白)是一个泛癌种的靶点。WT1 蛋白是一种转录因子,通常不在成人组织中表达,但会在大量癌症以及某些癌症干细胞中重新出现。在2009年美国国家癌症研究所(NCI)发布肿瘤抗原报告里,WT1为排名首位的免疫疗法肿瘤抗原,有望治疗20多种癌症。时过境迁,如今免疫疗法的第一靶点毫无疑问是PD-1和PD-L1这对双胞胎兄弟,但WT1的优质潜力仍然毋庸置疑。然而WT1不是一个容易触及的靶点,不能用小分子药物治疗,抗体也无法靠近它,用Sellas官网上的话说就是:传统方法尚未被证明可以成功靶向WT1蛋白。但它可以被免疫系统识别,特别是CD4+T细胞和CD8+T细胞。GPS最初由纪念斯隆-凯特琳癌症中心(MSK)开发,2014年9月4日Sellas与MSK签订了合作协议,获得了GPS的全球开发和商业化独家许可。不得不说,美国医疗产业,在产、学合作及成果转化上确实非常先进。Sellas接手之后,把GPS做成了全球进展最快的WT1癌症疫苗,除了单用之外,还在开发与其他免疫疗法联用的GPS+策略。一周前,2022年11月10日,Sellas还公布了GPS联合Keytruda(K药)在WT1阳性的复发或难治性铂类耐药晚期转移性卵巢癌的患者中1/2期临床研究的顶线结果。试验结果显示,患者的中位OS(mOS)为18.4个月,而此前单用PD-1治疗类似患者的mOS数据为13.8个月,延长4.6个月;中位无进展生存期(mPFS)为12周,单用PD-1为8周,延长50%。可以说效果还不错,因而在开篇提到的REGAL研究里,GPS有望获得超预期的疗效,被寄予厚望。然而对于财务状况不甚理想的Sellas来说,超出预期太多的疗效,反而有可能会成为一剂猛药,让“虚不受补”的Sellas陷入危机。2临床效果超预期,被迫更改试验方案REGAL研究的主要重点为总生存期(OS),试验组为GPS治疗组,对照组为接受最佳标准治疗(BAT)组。最新公布的结果来看,受试者的存活时间比他们之前对典型AML病程的假设要长的多。从试验设计来看,导致这种情况出现的原因可能有3个:1、入组人群选定的是经二线治疗后完全缓解(CR2)的AML患者,相当于抑制的是复发情况,如果在二线治疗里效果太好,患者有可能出现临床治愈的情况,进而大幅延长OS数据。2、对照组选择的并非安慰剂,而是“最佳标准治疗(BAT)”,最终选择的方案是艾伯维的维奈妥拉(Venclexta),如果对手超常发挥也可能会导致OS数据的延长。3、GPS的效果大幅超预期,拉长了平均OS。在2020年Sellas发表的三期临床启动消息中透露过,那些临床实现CR2的AML患者,mOS也仅有5个月;二期临床数据显示,GPS治疗组的mOS为21个月,而BAT组仅为5.4个月,GPS潜力巨大,因此再次超常发挥的可能性比较大。由于没办法实锤,只能继续进行试验,在与独立数据检测委员会协商后,试验方案进行了多项修改:入组患者从116名提高到125-140名患者;中期分析的目标死亡人数从80人减少到60人;最终分析的死亡人数从105人减少到80人;OS统计学显著性差异的标准更改为治疗组12.6个月,对照组8个月。中期分析预计在2023年底到2024年完成,最终分析预计在2024年底完成。出现这种情况按说是好事,虽然我们已经多次报道“对照组开挂”的情况,但大概率的原因还是GPS的疗效太好。不过Sellas的账上已经没钱了。3小公司经不起折腾据Sellas三季度财报显示,截止2022年9月30日,Sellas账面上还剩下月2130万美元的现金,而今年前三个季度的研发费用为1440万美元,净亏损为3220万美元,即便刨除对劲方医药的引进交易,仍然有超过2000万美元的亏损。在试验方案更改的情况下,研发支出只会增加。熬不过去了,本来已经要出结果了,现在又要多等一年多。不得已Sellas邀请思路迪医药参与了REGAL试验,从中国区这边增补大约20名患者。这也让中国患者参与GPS三期临床的时间大幅提前。按照原计划走的话,思路迪医药在今年10月才刚刚完成一期临床的首例患者入组,三期不知道要到什么时候。从投资者的角度来看,就好比原本借了你一笔钱,说好了2022年连本带利还,结果到了还款日,你说生意做的太大,资金周转不过来,手上没钱还了不说,还要再问我借一笔。确实有点难以接受。最大赢家可能会是思路迪。当初总金额2亿美元买来的GPS,效果越好自然是收益越大。目前思路迪医药正在准备第三次冲击港股IPO,3D189目前是其管线里的核心产品之一,如果最终效果非常理想,足够在二级市场引起一阵讨论。做药的都希望临床效果好,但好到这个程度确实让Sellas有点消受不起,在屏幕前仿佛看到喝了“十全大补汤”之后呲呲冒鼻血的Sellas。小公司经不起折腾了。4结语Sellas的危机,本质上还是资本市场过于冷淡引起的。医药研发难度太高,临床试验又变数太多,根本无法避免出现计划外的情况,这个时候团队的应变能力如何,找的CRO公司实力如何,都会成为药品开发能否成功的关键因素。除了这些自己能掌控的东西之外,背后的投资者是否专业,是否有足够的信心支持公司,也会成为重要的胜负手。而背后的投资人是谁,往往是在公司成立之时就已经定好了,技术出身的创业者们在做决定时,多花点时间思考,或许会成为关键时刻的伏笔。参考资料:https://www.fiercebiotech.com/biotech/sellas-retunes-leukemia-trial-patients-live-longer-expected-biotechs-drug-behind-survivalSellas官网肿瘤疫苗:醉里挑灯看剑,谁能率先突围?其他公开资料封面图/摘要图:网络版权声明/免责声明本文为原创内容,版权归作者所有。仅供感兴趣的个人谨慎参考,非商用,非医用、非投资用。欢迎朋友们批评指正!衷心感谢!文中图片、视频或为药时代购买的授权正版作品,或来自微信公共图片库,或取自公司官网/网络根据CC0协议使用,版权归拥有者。任何问题,请与我们联系(电话:13651980212。微信:27674131。邮箱:contact@drugtimes.cn)。衷心感谢!谢雨礼博士 | 未来十年新药研发,我们要做什么?MNC对「法案」的反击:礼来终止小分子药管线,AZ可能再也不在美国推出新抗癌药了...推荐阅读引领数字化转型!勃林格殷格翰荣膺2022上海国际生物医药产业周“数字化转型先锋企业”!MNC对「法案」的反击:礼来终止小分子药管线,AZ可能再也不在美国推出新抗癌药了...重磅资讯!亚硝胺潘多拉魔盒已经打开;美国最高法院拒绝受理BMS诉吉利徳的CAR-T专利侵权案;NIH加强临床试验结果公开管理谢雨礼博士 | 未来十年新药研发,我们要做什么?工艺表征中缩小模型的建立和确认前赛诺菲CEO上任渤健,曾被董事会一致票走,二者能否重振雄风?药时代BD-044项目 | 第三代linker技术ADC新药 靶向Claudin18.2 海外公司寻求中国合作伙伴CDMO内容分享 | 生物药无菌灌装生产,干货一网打尽!居安思危?临床数据一路向好,股价也大涨,但还是要裁员...股价仅剩峰值的2%!终止开发癌症免疫新疗法,陈列平也挡不住科学的规律...点击这里,与~20万+同药们喜相逢!
免疫疗法疫苗临床3期
分析
对领域进行一次全面的分析。
登录
或
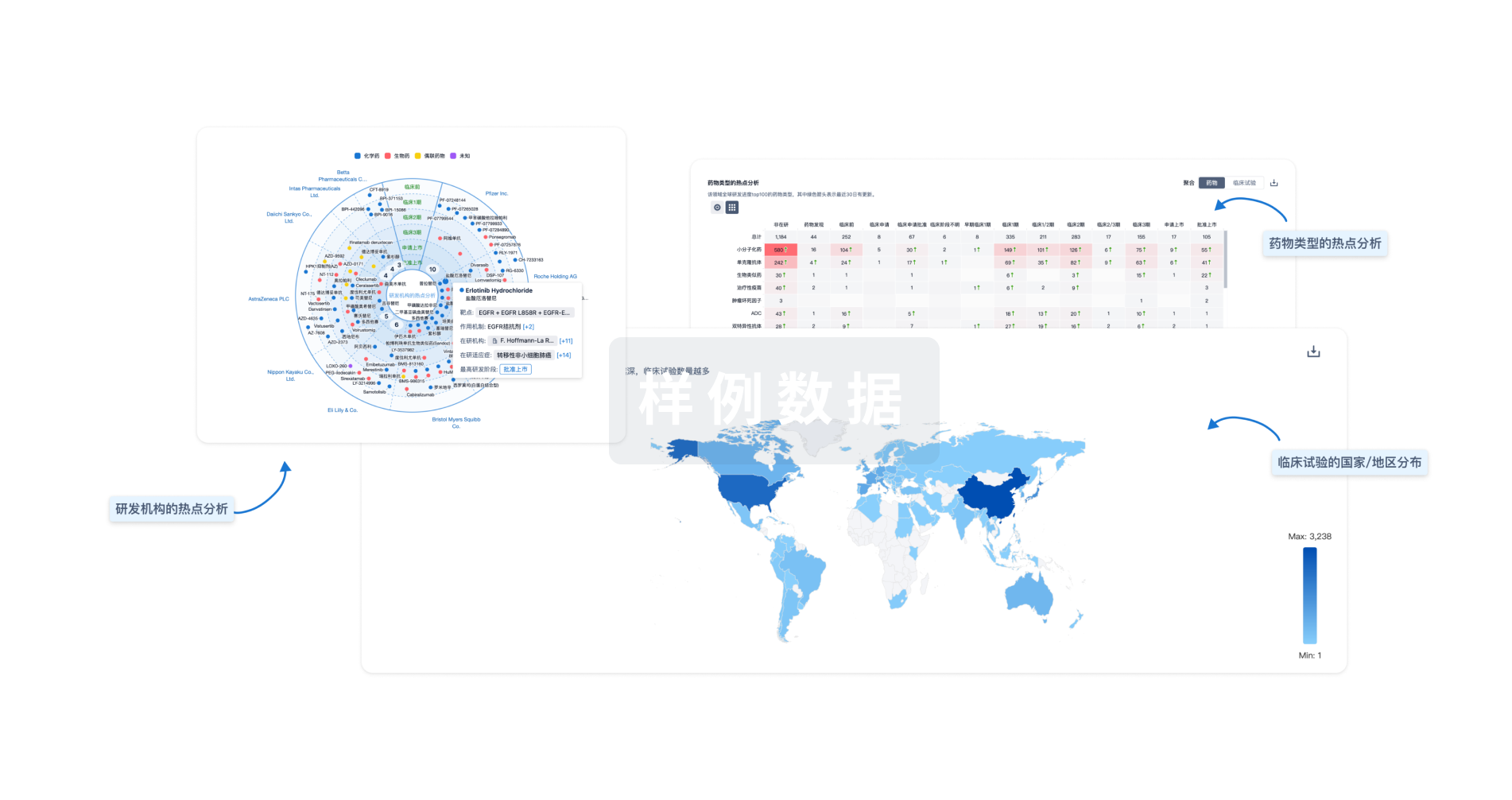
标准版
¥16800
元/账号/年
新药情报库 | 省钱又好用!
立即使用
来和芽仔聊天吧
立即开始免费试用!
智慧芽新药情报库是智慧芽专为生命科学人士构建的基于AI的创新药情报平台,助您全方位提升您的研发与决策效率。
立即开始数据试用!
智慧芽新药库数据也通过智慧芽数据服务平台,以API或者数据包形式对外开放,助您更加充分利用智慧芽新药情报信息。
生物序列数据库
生物药研发创新
免费使用
化学结构数据库
小分子化药研发创新
免费使用